Fuel ( IF 6.7 ) Pub Date : 2021-02-05 , DOI: 10.1016/j.fuel.2021.120217 Runzhao Li , Jose Martin Herreros , Athanasios Tsolakis , Wenzhao Yang
|
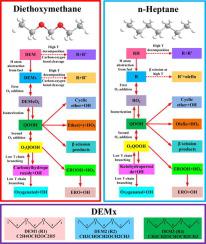
Diethoxymethane (DEM) is a carbon–neutral fuel with high cetane number (57.3). A detailed chemical kinetic mechanism for DEM oxidation covering low and high temperature reactions is first developed in this work. The reaction scheme and rate rules of DEM sub-mechanism are determined by the analogy method to n-heptane. Aramco 3.0 mechanism is used as a base mechanism to consider C0-C4 fuels while dimethoxymethane mechanism is included to ensure the mechanism compatibility and rate rule consistency. Thermodynamic and transport properties of new species in DEM sub-mechanism are computed by the methods of group additivity and properties correlation. The mechanism is validated against ignition delay times and premixed laminar flame speed measured by shock tube, rapid compression machine and spherical flame in combustion vessel. The verification covers a pressure range of 2~30 bar, an equivalence ratio range of 0.5~2.0, a temperature range of 540~1371 K. A satisfactory agreement between the experimental and computed results is observed, supporting the proposed reaction scheme and rate rules. Comparison of the ignition delay times between DEM and n-heptane indicates: (i) DEM is more reactive at low temperature (500~670 K) than n-heptane which favors low temperature combustion mode. (ii) DEM ignition delay times demonstrate monotonous temperature dependence at the full temperature regime but it is relatively independent of temperature at intermediate temperature (620~960 K). Therefore, a negative temperature coefficient (NTC) behavior is not observed in most conditions. (iii) DEM may not be an efficient chemical ignition source compared to n-heptane due to insufficient temperature increases and active radical accumulation.
中文翻译:

二乙氧基甲烷氧化的化学动力学模型:碳中性燃料
二乙氧基甲烷(DEM)是十六烷值高的碳中和燃料(57.3)。这项工作首先开发了涵盖低温和高温反应的DEM氧化的详细化学动力学机理。通过对正庚烷的类比法确定DEM亚机理的反应方案和速率规律。Aramco 3.0机制被用作考虑C0-C4燃料的基础机制,而包括二甲氧基甲烷机制以确保机制兼容性和速率规则一致性。通过群可加和性质相关的方法计算了DEM子机理中新物种的热力学和传递性质。该机构针对点火延迟时间和通过冲击管,快速压缩机以及燃烧容器中的球形火焰测量的预混合层流火焰速度进行了验证。验证涵盖2〜30 bar的压力范围,0.5〜2.0的当量比范围,540〜1371 K的温度范围。实验结果和计算结果之间存在令人满意的一致性,支持了拟议的反应方案和速率规则。比较DEM和正庚烷的点火延迟时间表明:(i)DEM在低温(500〜670 K)下比正庚烷更具反应性,这有利于低温燃烧模式。(ii)DEM点火延迟时间在整个温度范围内表现出单调的温度依赖性,但相对独立于中间温度(620〜960 K)的温度。因此,在大多数情况下都没有观察到负温度系数(NTC)行为。

































 京公网安备 11010802027423号
京公网安备 11010802027423号