当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalyst-free synthesis of 3,1-benzoxathiin-4-ones/1,3-benzodioxin-4-ones
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2021-1-27 , DOI: 10.1039/d0ob02543g Sengodagounder Muthusamy 1, 2, 3, 4 , Muthukumar Malarvizhi 1, 2, 3, 4 , Eringathodi Suresh 4, 5, 6, 7
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2021-1-27 , DOI: 10.1039/d0ob02543g Sengodagounder Muthusamy 1, 2, 3, 4 , Muthukumar Malarvizhi 1, 2, 3, 4 , Eringathodi Suresh 4, 5, 6, 7
Affiliation
|
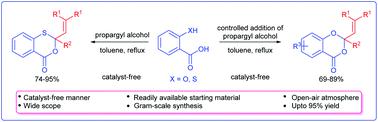
An unambiguous and precise method for the synthesis of 3,1-benzoxathiin-4-ones/1,3-benzodioxin-4-ones by the reaction of propargylic alcohols and salicylic/thiosalicylic acids under a catalyst-free and open-air atmosphere is described. This strategy is found to be quite general using various 2-mercapto and 2-hydroxybenzoic acids providing benzoxathiinones/benzodioxinones in good yields.
中文翻译:

无催化剂合成3,1-苯并恶臭素-4-酮/ 1,3-苯并二恶英-4-酮
在无催化剂的露天气氛下,通过炔丙醇与水杨酸/硫代水杨酸的反应,合成3,1-苯并沙硫辛-4-酮/ 1,3-苯并二恶英-4-酮的准确而精确的方法是描述。发现该策略使用各种2-巯基和2-羟基苯甲酸以提供高收率的苯并二氢噻吩酮/苯并二恶英酮是相当普遍的。
更新日期:2021-02-04
中文翻译:

无催化剂合成3,1-苯并恶臭素-4-酮/ 1,3-苯并二恶英-4-酮
在无催化剂的露天气氛下,通过炔丙醇与水杨酸/硫代水杨酸的反应,合成3,1-苯并沙硫辛-4-酮/ 1,3-苯并二恶英-4-酮的准确而精确的方法是描述。发现该策略使用各种2-巯基和2-羟基苯甲酸以提供高收率的苯并二氢噻吩酮/苯并二恶英酮是相当普遍的。































 京公网安备 11010802027423号
京公网安备 11010802027423号