当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Importance of Engineered and Learned Molecular Representations in Predicting Organic Reactivity, Selectivity, and Chemical Properties
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2021-02-03 , DOI: 10.1021/acs.accounts.0c00745 Liliana C. Gallegos 1 , Guilian Luchini 1 , Peter C. St. John 2 , Seonah Kim 2 , Robert S. Paton 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2021-02-03 , DOI: 10.1021/acs.accounts.0c00745 Liliana C. Gallegos 1 , Guilian Luchini 1 , Peter C. St. John 2 , Seonah Kim 2 , Robert S. Paton 1
Affiliation
|
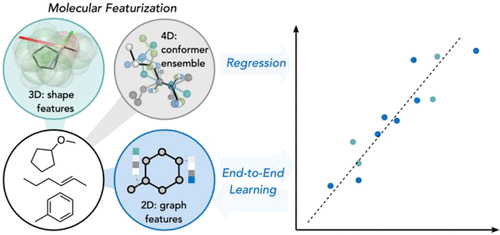
Machine-readable chemical structure representations are foundational in all attempts to harness machine learning for the prediction of reactivities, selectivities, and chemical properties directly from molecular structure. The featurization of discrete chemical structures into a continuous vector space is a critical phase undertaken before model selection, and the development of new ways to quantitatively encode molecules is an active area of research. In this Account, we highlight the application and suitability of different representations, from expert-guided “engineered” descriptors to automatically “learned” features, in different prediction tasks relevant to organic and organometallic chemistry, where differing amounts of training data are available. These tasks include statistical models of stereo- and enantioselectivity, thermochemistry, and kinetics developed using experimental and quantum chemical data.
中文翻译:

工程和学习的分子表示法在预测有机反应性,选择性和化学性质中的重要性
机器可读的化学结构表示形式是所有尝试利用机器学习直接从分子结构预测反应性,选择性和化学性质的基础。将离散化学结构特征化为连续向量空间是选择模型之前的关键阶段,开发定量编码分子的新方法是研究的活跃领域。在此帐户中,我们将重点介绍在有机和有机金属化学相关的不同预测任务中,从专家指导的“工程化”描述符到自动“学习”特征的不同表示形式的适用性和适用性,其中可获得不同数量的培训数据。这些任务包括立体和对映选择性,热化学,
更新日期:2021-02-16
中文翻译:

工程和学习的分子表示法在预测有机反应性,选择性和化学性质中的重要性
机器可读的化学结构表示形式是所有尝试利用机器学习直接从分子结构预测反应性,选择性和化学性质的基础。将离散化学结构特征化为连续向量空间是选择模型之前的关键阶段,开发定量编码分子的新方法是研究的活跃领域。在此帐户中,我们将重点介绍在有机和有机金属化学相关的不同预测任务中,从专家指导的“工程化”描述符到自动“学习”特征的不同表示形式的适用性和适用性,其中可获得不同数量的培训数据。这些任务包括立体和对映选择性,热化学,

































 京公网安备 11010802027423号
京公网安备 11010802027423号