Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 4.9 ) Pub Date : 2021-02-04 , DOI: 10.1016/j.colsurfa.2021.126279 Ridhima Chadha , Abhishek Das , Anil K. Debnath , Sudhir Kapoor , Nandita Maiti
|
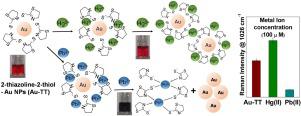
A novel 2-thiazoline-2-thiol functionalized gold (Au-TT) nanosensor was developed for the selective and sensitive detection of toxic heavy metal ions, Hg(II) and Pb(II) by colorimetry, and for studying its competitive surface reactivity using SERS and XPS. The detection mechanism and surface reactivity is based on competitive binding affinity of thiocarbonyl S or thiazoline ring N/S atom with the metal ions and the nanoparticles (NPs). Due to differences in the binding affinities of the metal ions towards the active binding sites of TT, addition of different metal ions resulted in variation of colour and SERS spectral features. Of the various metal ions viz. Cu(II), Cd(II), Co(II), Zn(II), Ni(II), Hg(II), Pb(II), Ca(II), Fe(II), Mn(II) and Sn(II), only Hg(II) and Pb(II) showed distinctive colorimetric and SERS spectral response that was quantified using XPS. This study, thus, realizes a selective and sensitive visual based nanosensor for Hg(II) and Pb(II) with limit of detection (LOD) of ∼ 0.1 ppm. SERS revealed the formation of Hg(TT)2 and Pb(TT)2 complexes on the Au NPs surface with the former remaining bonded to the NPs, resulting in enhanced Raman intensity for 1026 cm-1 band, while the latter gets desorbed from the surface leading to reduced SERS intensity.
中文翻译:

2-噻唑啉-2-硫醇官能化的金纳米颗粒,用于检测重金属,Hg(II)和Pb(II)并探测其竞争性表面反应性:比色,表面增强拉曼散射(SERS)和X射线光电子能谱(XPS) )研究
开发了一种新型的2-噻唑啉-2-硫醇官能化金(Au-TT)纳米传感器,用于通过比色法选择性和灵敏地检测有毒重金属离子Hg(II)和Pb(II),并研究其竞争性表面反应性使用SERS和XPS。检测机理和表面反应性基于硫代羰基S或噻唑啉环N / S原子与金属离子和纳米颗粒(NP)的竞争结合亲和力。由于金属离子对TT的活性结合位点的结合亲和力不同,因此添加不同的金属离子会导致颜色和SERS光谱特征发生变化。在各种金属离子中。Cu(II),Cd(II),Co(II),Zn(II),Ni(II),Hg(II),Pb(II),Ca(II),Fe(II),Mn(II)和锡(II)只有Hg(II)和Pb(II)表现出独特的比色和SERS光谱响应,这些响应使用XPS进行了量化。因此,本研究实现了一种针对Hg(II)和Pb(II)的选择性,灵敏的基于视觉的纳米传感器,其检出限(LOD)约为0.1 ppm。SERS揭示了Hg(TT)的形成Au NPs表面上的2和Pb(TT)2络合物保留前者与NPs的结合,导致拉曼强度在1026 cm -1谱带增强,而后者从表面解吸,导致SERS强度降低。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号