Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Alcohol-Assisted Hydrogenation of Carbon Monoxide to Methanol Using Molecular Manganese Catalysts
JACS Au ( IF 8.5 ) Pub Date : 2021-01-25 , DOI: 10.1021/jacsau.0c00091 Akash Kaithal 1 , Christophe Werlé 1, 2 , Walter Leitner 1, 3
JACS Au ( IF 8.5 ) Pub Date : 2021-01-25 , DOI: 10.1021/jacsau.0c00091 Akash Kaithal 1 , Christophe Werlé 1, 2 , Walter Leitner 1, 3
Affiliation
|
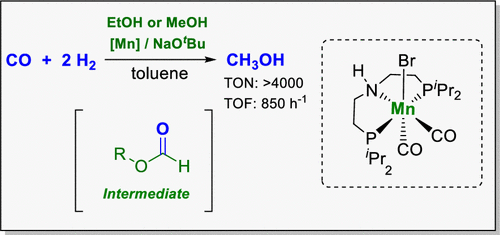
Alcohol-assisted hydrogenation of carbon monoxide (CO) to methanol was achieved using homogeneous molecular complexes. The molecular manganese complex [Mn(CO)2Br[HN(C2H4PiPr2)2]] ([HN(C2H4PiPr2)2] = MACHO-iPr) revealed the best performance, reaching up to turnover number = 4023 and turnover frequency 857 h–1 in EtOH/toluene as solvent under optimized conditions (T = 150 °C, p(CO/H2) = 5/50 bar, t = 8–12 h). Control experiments affirmed that the reaction proceeds via formate ester as the intermediate, whereby a catalytic amount of base was found to be sufficient to mediate its formation from CO and the alcohol in situ. Selectivity for methanol formation reached >99% with no accumulation of the formate ester. The reaction was demonstrated to work with methanol as the alcohol component, resulting in a reactive system that allows catalytic “breeding” of methanol without any coreagents.
中文翻译:

使用分子锰催化剂醇辅助一氧化碳加氢制甲醇
使用均相分子复合物实现了醇辅助一氧化碳 (CO) 氢化为甲醇。分子锰配合物 [Mn(CO) 2 Br [HN(C 2 H 4 Pi Pr 2 ) 2 ] ] ([HN(C 2 H 4 Pi Pr 2 ) 2 ] = MACHO- i Pr) 显示了最好的性能,在优化条件下( T = 150 °C,p (CO/H 2 ) = 5/50 bar,t = 8–12,以乙醇/甲苯为溶剂,周转数可达 4023,周转频率可达 857 h –1 H)。对照实验证实该反应通过甲酸酯作为中间体进行,由此发现催化量的碱足以介导其由CO和醇原位形成。甲醇形成的选择性达到>99%,且没有甲酸酯的积累。该反应被证明可以使用甲醇作为醇组分,从而形成一个反应系统,无需任何助剂即可催化“繁殖”甲醇。
更新日期:2021-02-22
中文翻译:

使用分子锰催化剂醇辅助一氧化碳加氢制甲醇
使用均相分子复合物实现了醇辅助一氧化碳 (CO) 氢化为甲醇。分子锰配合物 [Mn(CO) 2 Br [HN(C 2 H 4 Pi Pr 2 ) 2 ] ] ([HN(C 2 H 4 Pi Pr 2 ) 2 ] = MACHO- i Pr) 显示了最好的性能,在优化条件下( T = 150 °C,p (CO/H 2 ) = 5/50 bar,t = 8–12,以乙醇/甲苯为溶剂,周转数可达 4023,周转频率可达 857 h –1 H)。对照实验证实该反应通过甲酸酯作为中间体进行,由此发现催化量的碱足以介导其由CO和醇原位形成。甲醇形成的选择性达到>99%,且没有甲酸酯的积累。该反应被证明可以使用甲醇作为醇组分,从而形成一个反应系统,无需任何助剂即可催化“繁殖”甲醇。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号