当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Transfer Hydrogenation of α-Substituted-β-Keto Carbonitriles via Dynamic Kinetic Resolution
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-02-02 , DOI: 10.1021/jacs.0c13273
Fangyuan Wang 1, 2 , Tilong Yang 2 , Ting Wu 3 , Long-Sheng Zheng 2 , Congcong Yin 2 , Yongjie Shi 2 , Xiang-Yu Ye 2 , Gen-Qiang Chen , Xumu Zhang
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-02-02 , DOI: 10.1021/jacs.0c13273
Fangyuan Wang 1, 2 , Tilong Yang 2 , Ting Wu 3 , Long-Sheng Zheng 2 , Congcong Yin 2 , Yongjie Shi 2 , Xiang-Yu Ye 2 , Gen-Qiang Chen , Xumu Zhang
Affiliation
|
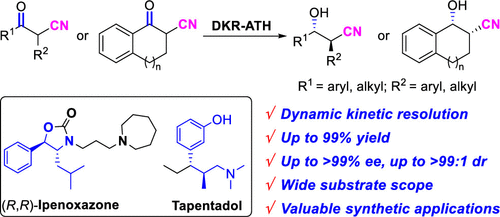
A catalytic protocol for the enantio- and diastereoselective reduction of α-substituted-β-keto carbonitriles is described. The reaction involves a DKR-ATH process with the simultaneous construction of β-hydroxy carbonitrile scaffolds with two contiguous stereogenic centers. A wide range of α-substituted-β-keto carbonitriles were obtained in high yields (94%–98%) and excellent enantio- and diastereoselectivities (up to >99% ee, up to >99:1 dr). The origin of the diastereoselectivity was also rationalized by DFT calculations. Furthermore, this methodology offers rapid access to the pharmaceutical intermediates of Ipenoxazone and Tapentadol.
中文翻译:

动态动力学拆分法对α-取代-β-酮腈进行不对称加氢
描述了用于α-取代的-β-酮腈的对映体和非对映体选择性还原的催化方案。该反应涉及DKR-ATH方法,该方法同时构建具有两个连续的立体异构中心的β-羟基甲腈支架。以高产率(94%–98%)和出色的对映和非对映选择性(高达> 99%ee,高达> 99:1 dr)获得了多种α-取代-β-酮腈。非对映选择性的起源也通过DFT计算得到了合理化。此外,这种方法可以快速获得异戊ox酮和他喷他多的药物中间体。
更新日期:2021-02-17
中文翻译:

动态动力学拆分法对α-取代-β-酮腈进行不对称加氢
描述了用于α-取代的-β-酮腈的对映体和非对映体选择性还原的催化方案。该反应涉及DKR-ATH方法,该方法同时构建具有两个连续的立体异构中心的β-羟基甲腈支架。以高产率(94%–98%)和出色的对映和非对映选择性(高达> 99%ee,高达> 99:1 dr)获得了多种α-取代-β-酮腈。非对映选择性的起源也通过DFT计算得到了合理化。此外,这种方法可以快速获得异戊ox酮和他喷他多的药物中间体。

































 京公网安备 11010802027423号
京公网安备 11010802027423号