当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, Crystal Structure, Optical Properties and Stability of New Bismuth‐Based Organic‐Inorganic Compounds (C6H9N2)aBibXc (X=Cl, Br, I)
ChemistrySelect ( IF 1.9 ) Pub Date : 2021-02-03 , DOI: 10.1002/slct.202004010 Xinru Hu 1 , Jilin Wang 1, 2, 3 , Wenhui Mao 1 , Guoyuan Zheng 1, 2 , Shuyi Mo 1, 2, 3 , Nan Tian 1, 2 , Bing Zhou 1, 2 , Fei Long 1, 2, 3 , Zhengguang Zou 1, 2
ChemistrySelect ( IF 1.9 ) Pub Date : 2021-02-03 , DOI: 10.1002/slct.202004010 Xinru Hu 1 , Jilin Wang 1, 2, 3 , Wenhui Mao 1 , Guoyuan Zheng 1, 2 , Shuyi Mo 1, 2, 3 , Nan Tian 1, 2 , Bing Zhou 1, 2 , Fei Long 1, 2, 3 , Zhengguang Zou 1, 2
Affiliation
|
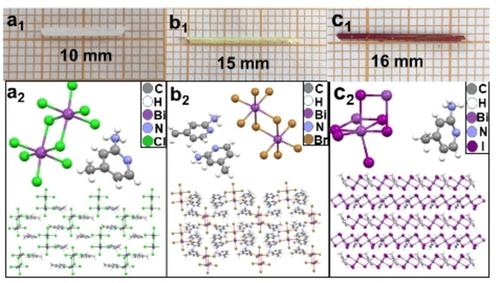
We report a series of high‐quality, low‐dimension, and bismuth‐based organic‐inorganic hybrid single crystals (C6H9N2)aBibXc (X=Cl, Br, I) fabricated via a simple hydrothermal method. The crystal structure, intermolecular interactions, morphology, chemical groups, bond characterization, and optical and thermal stability of the samples were detailed via X‐ray single crystal diffractomete, Hirshfeld surface analysis, field scanning electron microscopy (FSEM), fourier transform infrared spectrometer (FTIR), Raman, thermal gravimetric (TG), and Ultraviolet‐visible (UV‐vis) diffuse reflectance spectra. The results indicated that the (C6H9N2)2BiCl5 and (C6H9N2)2BiBr5 crystals displayed a monoclinic system with a P21/n space group at room temperature; the (C6H9N2)BiI4 crystal was P21/c. Meanwhile, the band gap could be continuously adjusted between 3.2 eV and 1.6 eV via the nature of halide ligands. The samples also have good thermal stability. The starting decomposition temperature of (C6H9N2)2BiCl5, (C6H9N2)2BiBr5, and (C6H9N2)BiI4 were 200 °C, 250 °C, and 300 °C, respectively. Therefore, this kind of organic‐inorganic hybrid compounds will be assumed to be a promising candidate for optical and electronic applications.
中文翻译:

新型铋基有机无机化合物(C6H9N2)aBibXc(X = Cl,Br,I)的合成,晶体结构,光学性质和稳定性
我们报告了通过简单的水热法制备的一系列高质量,低尺寸和基于铋的有机-无机杂化单晶(C 6 H 9 N 2)a Bi b X c(X = Cl,Br,I)方法。样品的晶体结构,分子间相互作用,形态,化学基团,键表征以及光学和热稳定性通过X射线单晶衍射仪,Hirshfeld表面分析,场扫描电子显微镜(FSEM),傅立叶变换红外光谱仪( FTIR),拉曼光谱,热重(TG)和紫外可见(UV-vis)漫反射光谱。结果表明(C 6 H 9N 2)2 BiCl 5和(C 6 H 9 N 2)2 BiBr 5晶体在室温下显示出具有P21 / n空间群的单斜晶系统。(C 6 H 9 N 2)BiI 4晶体为P21 / c。同时,带隙可以通过卤化物配体的性质在3.2 eV和1.6 eV之间连续调节。样品还具有良好的热稳定性。(C 6 H 9 N 2)2 BiCl 5的起始分解温度,(C 6 H9 N 2)2 BiBr 5和(C 6 H 9 N 2)BiI 4分别为200°C,250°C和300°C。因此,这种有机-无机杂化化合物将被认为是光学和电子应用的有前途的候选者。
更新日期:2021-02-03
中文翻译:

新型铋基有机无机化合物(C6H9N2)aBibXc(X = Cl,Br,I)的合成,晶体结构,光学性质和稳定性
我们报告了通过简单的水热法制备的一系列高质量,低尺寸和基于铋的有机-无机杂化单晶(C 6 H 9 N 2)a Bi b X c(X = Cl,Br,I)方法。样品的晶体结构,分子间相互作用,形态,化学基团,键表征以及光学和热稳定性通过X射线单晶衍射仪,Hirshfeld表面分析,场扫描电子显微镜(FSEM),傅立叶变换红外光谱仪( FTIR),拉曼光谱,热重(TG)和紫外可见(UV-vis)漫反射光谱。结果表明(C 6 H 9N 2)2 BiCl 5和(C 6 H 9 N 2)2 BiBr 5晶体在室温下显示出具有P21 / n空间群的单斜晶系统。(C 6 H 9 N 2)BiI 4晶体为P21 / c。同时,带隙可以通过卤化物配体的性质在3.2 eV和1.6 eV之间连续调节。样品还具有良好的热稳定性。(C 6 H 9 N 2)2 BiCl 5的起始分解温度,(C 6 H9 N 2)2 BiBr 5和(C 6 H 9 N 2)BiI 4分别为200°C,250°C和300°C。因此,这种有机-无机杂化化合物将被认为是光学和电子应用的有前途的候选者。

































 京公网安备 11010802027423号
京公网安备 11010802027423号