当前位置:
X-MOL 学术
›
Biotechnol. Appl. Bioc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
High production of d-psicose from d-fructose by immobilized whole recombinant Bacillus subtilis cells expressing d-psicose 3-epimerase from Agrobacterium tumefaciens
Biotechnology and Applied Biochemistry ( IF 3.2 ) Pub Date : 2021-02-03 , DOI: 10.1002/bab.2115 Jianwei Wang 1 , Jiandong Sun 1 , Hongqing Qi 1 , Liang Wang 1 , Jihui Wang 1 , Cheng Li 1
Biotechnology and Applied Biochemistry ( IF 3.2 ) Pub Date : 2021-02-03 , DOI: 10.1002/bab.2115 Jianwei Wang 1 , Jiandong Sun 1 , Hongqing Qi 1 , Liang Wang 1 , Jihui Wang 1 , Cheng Li 1
Affiliation
|
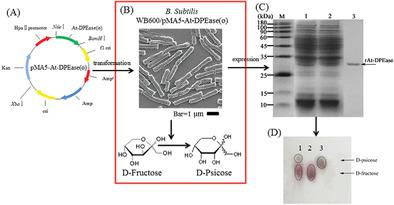
d-Psicose 3-epimerase (DPEase) can catalyze the isomerization of d-fructose to be rare sugar d-psicose, which has wide application prospects in the food and medical fields. In this study, the DPEase gene from Agrobacterium tumefaciens was constructed into plasmid pMA5, and was successfully expressed in the host Bacillus subtilis WB600 (B. subtilis). After optimization of the fermentation conditions, whole recombinant B. subtilis WB600/pMA5-At-DEPase(O) cells produced d-psicose from d-fructose with a conversion rate of 29.01 ± 0.19%, which could be used for the efficient synthesis of d-psicose. To further improve the whole recombinant B. subtilis application, B. subtilis cells were immobilized onto a gel bead biocatalyst by Ca-alginate. After optimization of the biotransformation conditions, the conversion rate of the immobilized biocatalyst reached 20.74 ± 0.39%, which was lower than the free cells. However, the results showed that the immobilized biocatalyst had higher thermal/pH stability and storability, and the gel beads could be recycled for at least six batches. The results showed that the amount of d-psicose generated reached 32.83 ± 2.56 g/L with the immobilized biocatalyst after six times biotransformation, whereas the free cells produced only approximately 10.44 ± 0.07 g/L. The results showed that immobilized recombinant B. subtilis cells are promising to use for the efficient synthesis of d-psicose.
中文翻译:

通过表达来自根癌农杆菌的 d-阿洛酮糖 3-差向异构酶的固定化完整重组枯草芽孢杆菌细胞从 d-果糖高产 d-阿洛酮糖
d-阿洛酮糖3-差向异构酶(DPEase)可催化d-果糖异构化为稀有糖d-阿洛酮糖,在食品和医药领域具有广阔的应用前景。本研究将来自根癌农杆菌的DPEase基因构建到质粒pMA5中,并在宿主枯草芽孢杆菌WB600( B. subtilis )中成功表达。优化发酵条件后,全重组枯草芽孢杆菌WB600/pMA5-At-DEPase(O)细胞由d-果糖产生d-阿洛酮糖,转化率为29.01±0.19%,可用于高效合成果糖。d-阿洛酮糖。为了进一步改进整个重组枯草芽孢杆菌的应用,枯草芽孢杆菌细胞通过海藻酸钙固定在凝胶珠生物催化剂上。优化生物转化条件后,固定化生物催化剂的转化率达到20.74±0.39%,低于游离细胞。然而,结果表明,固定化生物催化剂具有更高的热/pH稳定性和储存性,凝胶珠粒至少可以循环使用六批。结果表明,固定化生物催化剂经六次生物转化后,产生的d-阿洛酮糖量达到32.83±2.56 g/L,而游离细胞仅产生约10.44±0.07 g/L。结果表明,固定化重组枯草芽孢杆菌细胞有望用于高效合成d-阿洛酮糖。
更新日期:2021-02-03
中文翻译:

通过表达来自根癌农杆菌的 d-阿洛酮糖 3-差向异构酶的固定化完整重组枯草芽孢杆菌细胞从 d-果糖高产 d-阿洛酮糖
d-阿洛酮糖3-差向异构酶(DPEase)可催化d-果糖异构化为稀有糖d-阿洛酮糖,在食品和医药领域具有广阔的应用前景。本研究将来自根癌农杆菌的DPEase基因构建到质粒pMA5中,并在宿主枯草芽孢杆菌WB600( B. subtilis )中成功表达。优化发酵条件后,全重组枯草芽孢杆菌WB600/pMA5-At-DEPase(O)细胞由d-果糖产生d-阿洛酮糖,转化率为29.01±0.19%,可用于高效合成果糖。d-阿洛酮糖。为了进一步改进整个重组枯草芽孢杆菌的应用,枯草芽孢杆菌细胞通过海藻酸钙固定在凝胶珠生物催化剂上。优化生物转化条件后,固定化生物催化剂的转化率达到20.74±0.39%,低于游离细胞。然而,结果表明,固定化生物催化剂具有更高的热/pH稳定性和储存性,凝胶珠粒至少可以循环使用六批。结果表明,固定化生物催化剂经六次生物转化后,产生的d-阿洛酮糖量达到32.83±2.56 g/L,而游离细胞仅产生约10.44±0.07 g/L。结果表明,固定化重组枯草芽孢杆菌细胞有望用于高效合成d-阿洛酮糖。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号