当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Spatiotemporal Concurrent Liberation of Cytotoxins from Dual-Prodrug Nanomedicine for Synergistic Antitumor Therapy
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-02-01 , DOI: 10.1021/acsami.0c21422
Liuwei Zhang 1, 2 , Ming Qian 2 , Hongyan Cui 2 , Shuang Zeng 2 , Jingyun Wang 1, 2 , Qixian Chen 2, 3
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-02-01 , DOI: 10.1021/acsami.0c21422
Liuwei Zhang 1, 2 , Ming Qian 2 , Hongyan Cui 2 , Shuang Zeng 2 , Jingyun Wang 1, 2 , Qixian Chen 2, 3
Affiliation
|
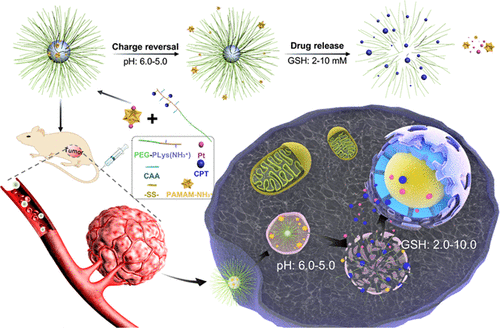
Nanomedicine developed to date by means of directly encapsulating cytotoxins suffers from crucial drawbacks, including premature release and detoxification prior to arrival at pharmaceutics targets. To these respects, redox-responsive polymeric prodrugs of platinum (Pt) and camptothecin (CPT), selectively and concomitantly activated in the cytoplasm, were elaborated in manufacture of dual prodrug nanomedicine. Herein, multiple CPTs were conjugated to poly(lysine) (PLys) segments of block copolymeric poly(ethylene glycol) (PEG)-PLys through the redox responsive disulfide linkage [PEG-PLys(ss-CPT)] followed by reversible conversion of amino groups from PLys into carboxyl groups based on their reaction with cis-aconitic anhydride [PEG-PLys(ss-CPT&CAA)]. On the other hand, Pt(IV) in conjugation with dendritic polyamindoamine [(G3-PAMAM-Pt(IV)] was synthesized for electrostatic complexation with PEG-PLys(ss-CPT&CAA) into dual prodrug nanomedicine. Subsequent investigations proved that the elaborated nanomedicine could sequentially respond to intracellular chemical potentials to overcome a string of predefined biological barriers and facilitate intracellular trafficking. Notably, PEG-PLys(ss-CPT&CAA) capable of responding to the acidic endosomal microenvironment for transformation into endosome-disruptive PEG-PLys(ss-CPT), as well as release of G3-PAMAM-Pt(IV) from nanomedicine, prompted transclocation of therapeutic payloads from endosomes into cytosols. Moreover, concurrent activation and liberation of cytotoxic CPT and Pt(II) owing to their facile responsiveness to the cytoplasmic reducing microenvironment have demonstrated overwhelming cytotoxic potencies. Eventually, systemic administration of the dual prodrug construct exerted potent tumor suppression efficacy in treatment of intractable solid breast adenocarcinoma, as well as an appreciable safety profile. The present study illustrated the first example of nanomedicine with a dual prodrug motif, precisely and concomitantly activated by the same subcellular stimuli before approaching pharmaceutic action targets, thus shedding important implication in development of advanced nanomedicine to seek maximized pharmaceutic outcomes.
中文翻译:

时空并发从双重药物纳米药物中协同释放细胞毒素,用于协同抗肿瘤治疗
迄今为止,通过直接包封细胞毒素而开发的纳米药物具有严重的缺点,包括在到达药物靶标之前过早释放和解毒。就这些方面而言,在双重前药纳米药物的制造中,精心设计了在细胞质中选择性并伴随活化的铂(Pt)和喜树碱(CPT)的氧化还原反应性聚合物前药。在此,多个CPT通过氧化还原响应性二硫键[PEG-PLys(ss-CPT)]与嵌段共聚物聚(乙二醇)-(PEG)-PLys的聚(赖氨酸)(PLys)链段共轭,然后氨基的可逆转化PLys与顺式反应后从PLys变成羧基-乙酸酐[PEG-PLys(ss-CPT&CAA)]。另一方面,将Pt(IV)与树状多胺[[G3-PAMAM-Pt(IV)]结合,用于与PEG-PLys(ss-CPT&CAA)的静电复合成双重前药纳米药物,随后的研究证明了该方法的复杂性。纳米药物可以顺序响应细胞内的化学潜能,克服一系列预定的生物学障碍并促进细胞内运输,尤其是能够对酸性内体微环境产生响应并转化为破坏内体的PEG-PLys的PEG-PLys(ss-CPT&CAA)。 -CPT)以及G3-PAMAM-Pt(IV)从纳米药物中释放出来,促使治疗有效载荷从内体转移到细胞质中。细胞毒性CPT和Pt(II)对细胞质减少的微环境反应灵敏,因此可同时激活和释放,这显示出压倒性的细胞毒性潜能。最终,双重前药构建体的全身给药在治疗顽固性乳腺腺癌中发挥了有效的肿瘤抑制功效,并且具有明显的安全性。本研究说明了具有双重前药基序的纳米药物的第一个实例,该纳米药物在接近药物作用目标之前被相同的亚细胞刺激精确并伴随地激活,因此在开发先进的纳米药物以寻求最大化的药物结果方面具有重要意义。最终,双重前药构建体的全身给药在治疗顽固性乳腺腺癌中发挥了有效的肿瘤抑制功效,并且具有明显的安全性。本研究说明了具有双重前药基序的纳米药物的第一个实例,该纳米药物在接近药物作用目标之前被相同的亚细胞刺激精确地并伴随地激活,因此在开发先进的纳米药物以寻求最大化的药物结果方面具有重要意义。最终,双重前药构建体的全身给药在治疗顽固性乳腺腺癌中发挥了有效的肿瘤抑制功效,并且具有明显的安全性。本研究说明了具有双重前药基序的纳米药物的第一个实例,该纳米药物在接近药物作用目标之前被相同的亚细胞刺激精确并伴随地激活,因此在开发先进的纳米药物以寻求最大化的药物结果方面具有重要意义。
更新日期:2021-02-10
中文翻译:

时空并发从双重药物纳米药物中协同释放细胞毒素,用于协同抗肿瘤治疗
迄今为止,通过直接包封细胞毒素而开发的纳米药物具有严重的缺点,包括在到达药物靶标之前过早释放和解毒。就这些方面而言,在双重前药纳米药物的制造中,精心设计了在细胞质中选择性并伴随活化的铂(Pt)和喜树碱(CPT)的氧化还原反应性聚合物前药。在此,多个CPT通过氧化还原响应性二硫键[PEG-PLys(ss-CPT)]与嵌段共聚物聚(乙二醇)-(PEG)-PLys的聚(赖氨酸)(PLys)链段共轭,然后氨基的可逆转化PLys与顺式反应后从PLys变成羧基-乙酸酐[PEG-PLys(ss-CPT&CAA)]。另一方面,将Pt(IV)与树状多胺[[G3-PAMAM-Pt(IV)]结合,用于与PEG-PLys(ss-CPT&CAA)的静电复合成双重前药纳米药物,随后的研究证明了该方法的复杂性。纳米药物可以顺序响应细胞内的化学潜能,克服一系列预定的生物学障碍并促进细胞内运输,尤其是能够对酸性内体微环境产生响应并转化为破坏内体的PEG-PLys的PEG-PLys(ss-CPT&CAA)。 -CPT)以及G3-PAMAM-Pt(IV)从纳米药物中释放出来,促使治疗有效载荷从内体转移到细胞质中。细胞毒性CPT和Pt(II)对细胞质减少的微环境反应灵敏,因此可同时激活和释放,这显示出压倒性的细胞毒性潜能。最终,双重前药构建体的全身给药在治疗顽固性乳腺腺癌中发挥了有效的肿瘤抑制功效,并且具有明显的安全性。本研究说明了具有双重前药基序的纳米药物的第一个实例,该纳米药物在接近药物作用目标之前被相同的亚细胞刺激精确并伴随地激活,因此在开发先进的纳米药物以寻求最大化的药物结果方面具有重要意义。最终,双重前药构建体的全身给药在治疗顽固性乳腺腺癌中发挥了有效的肿瘤抑制功效,并且具有明显的安全性。本研究说明了具有双重前药基序的纳米药物的第一个实例,该纳米药物在接近药物作用目标之前被相同的亚细胞刺激精确地并伴随地激活,因此在开发先进的纳米药物以寻求最大化的药物结果方面具有重要意义。最终,双重前药构建体的全身给药在治疗顽固性乳腺腺癌中发挥了有效的肿瘤抑制功效,并且具有明显的安全性。本研究说明了具有双重前药基序的纳米药物的第一个实例,该纳米药物在接近药物作用目标之前被相同的亚细胞刺激精确并伴随地激活,因此在开发先进的纳米药物以寻求最大化的药物结果方面具有重要意义。





































 京公网安备 11010802027423号
京公网安备 11010802027423号