Tetrahedron Letters ( IF 1.5 ) Pub Date : 2021-02-02 , DOI: 10.1016/j.tetlet.2021.152843 Curt A. Dvorak , Jimmy Liang , Neelakandha S. Mani , Nicholas I. Carruthers
|
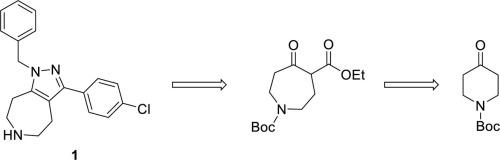
The synthesis of the 5-HT7 antagonist 1-benzyl-3-(4-chlorophenyl)-1,4,5,6,7,8-hexahydropyrazolo[3,4-d]azepine is described using a regioselective assembly of a pyrazole ring fused to an azepine ring. Two different approaches were examined for the construction of the fused pyrazole-azepine heterocyclic core. These were based on the timing and method of installation of the appended aryl ring and construction of the fused heterocycle. The team focused on a route that featured a palladium coupling reaction to introduce the aryl ring via a pyrazole triflate and a selective alkylation to set the position of benzyl moiety on the pyrazole nitrogen. This led to a scalable synthesis of 1 (JNJ-18038683) allowing the discovery team to select and advance a clinical candidate.
中文翻译:

稠合吡唑-氮杂杂环的区域选择性组装:5-HT 7拮抗剂1-苄基-3-(4-氯苯基)-1,4,5,6,7,8-六氢吡唑并[3,4- d ]氮杂的合成
5-HT 7拮抗剂1-苄基-3-(4-氯苯基)-1,4,5,6,7,8-六氢吡唑并[3,4- d ]氮杂的合成是使用a的区域选择性组装来描述的与吡咯环稠合的吡唑环。检查了两种不同的方法用于稠合吡唑-氮杂杂环核心的构建。这些基于附接的芳基环的安装时间和方法以及稠合杂环的构造。该团队着重研究了一种以钯偶联反应为特征的途径,该反应通过三氟甲磺酸吡唑引入芳环,并进行选择性烷基化以设定苄基在吡唑氮上的位置。这导致1的可扩展综合 (JNJ-18038683)允许发现小组选择并提升临床候选人。






























 京公网安备 11010802027423号
京公网安备 11010802027423号