当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An Iron(IV)–Oxo Intermediate Initiating l-Arginine Oxidation but Not Ethylene Production by the 2-Oxoglutarate-Dependent Oxygenase, Ethylene-Forming Enzyme
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-02-01 , DOI: 10.1021/jacs.0c10923 Rachelle A Copeland , Katherine M Davis , Tokufu Kent C Shoda , Elizabeth J Blaesi , Amie K Boal , Carsten Krebs , J Martin Bollinger
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-02-01 , DOI: 10.1021/jacs.0c10923 Rachelle A Copeland , Katherine M Davis , Tokufu Kent C Shoda , Elizabeth J Blaesi , Amie K Boal , Carsten Krebs , J Martin Bollinger
|
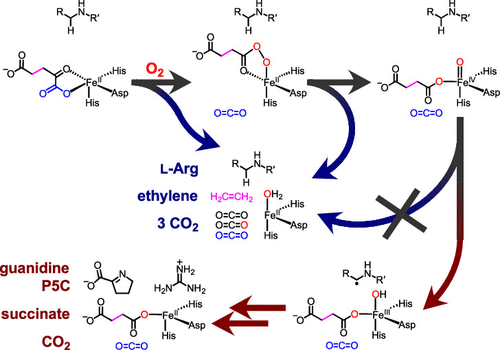
Ethylene-forming enzyme (EFE) is an ambifunctional iron(II)- and 2-oxoglutarate-dependent (Fe/2OG) oxygenase. In its major (EF) reaction, it converts carbons 1, 2, and 5 of 2OG to CO2 and carbons 3 and 4 to ethylene, a four-electron oxidation drastically different from the simpler decarboxylation of 2OG to succinate mediated by all other Fe/2OG enzymes. EFE also catalyzes a minor reaction, in which the normal decarboxylation is coupled to oxidation of l-arginine (a required activator for the EF pathway), resulting in its conversion to l-glutamate semialdehyde and guanidine. Here we show that, consistent with precedent, the l-Arg-oxidation (RO) pathway proceeds via an iron(IV)–oxo (ferryl) intermediate. Use of 5,5-[2H2]-l-Arg slows decay of the ferryl complex by >16-fold, implying that RO is initiated by hydrogen-atom transfer (HAT) from C5. That this large substrate deuterium kinetic isotope effect has no impact on the EF:RO partition ratio implies that the same ferryl intermediate cannot be on the EF pathway; the pathways must diverge earlier. Consistent with this conclusion, the variant enzyme bearing the Asp191Glu ligand substitution accumulates ∼4 times as much of the ferryl complex as the wild-type enzyme and exhibits a ∼40-fold diminished EF:RO partition ratio. The selective detriment of this nearly conservative substitution to the EF pathway implies that it has unusually stringent stereoelectronic requirements. An active-site, like-charge guanidinium pair, which involves the l-Arg substrate/activator and is unique to EFE among four crystallographically characterized l-Arg-modifying Fe/2OG oxygenases, may serve to selectively stabilize the transition state leading to the unique EF branch.
中文翻译:

铁 (IV)-氧代中间体通过 2-氧戊二酸依赖性加氧酶启动 l-精氨酸氧化,但不产生乙烯,乙烯形成酶
乙烯形成酶 (EFE) 是一种双功能铁 (II) 和 2-酮戊二酸依赖性 (Fe/2OG) 加氧酶。在其主要 (EF) 反应中,它将 2OG 的碳 1、2 和 5 转化为 CO 2,将碳 3 和 4 转化为乙烯,这是一种四电子氧化,与由所有其他 Fe 介导的 2OG 脱羧为琥珀酸盐的简单脱羧截然不同/2OG酶。EFE 还催化一个次要反应,其中正常的脱羧与l-精氨酸(EF 途径所需的活化剂)的氧化相结合,导致其转化为l-谷氨酸半醛和胍。在这里,我们表明,与先例一致,l -Arg-氧化(RO)途径通过铁(IV)-氧代(ferryl)中间体进行。使用 5,5-[ 2H 2 ]- l -Arg 将 Ferrel 复合物的衰变减慢 > 16 倍,这意味着 RO 是由来自 C5 的氢原子转移 (HAT) 引发的。这种大底物氘动力学同位素效应对 EF:RO 分配比没有影响,这意味着相同的 Ferrel 中间体不能出现在 EF 通路上;途径必须更早地分道扬镳。与该结论一致,带有 Asp191Glu 配体取代的变体酶积累的 Ferrel 复合物是野生型酶的 4 倍,EF:RO 分配比降低了 40 倍。这种对 EF 途径的近乎保守的替代的选择性损害意味着它具有异常严格的立体电子要求。一个活性位点,同电荷胍对,涉及l -Arg 底物/活化剂是 EFE 在四种晶体学特征的l -Arg 修饰 Fe/2OG 加氧酶中所独有的,可用于选择性地稳定导致独特 EF 分支的过渡态。
更新日期:2021-02-10
中文翻译:

铁 (IV)-氧代中间体通过 2-氧戊二酸依赖性加氧酶启动 l-精氨酸氧化,但不产生乙烯,乙烯形成酶
乙烯形成酶 (EFE) 是一种双功能铁 (II) 和 2-酮戊二酸依赖性 (Fe/2OG) 加氧酶。在其主要 (EF) 反应中,它将 2OG 的碳 1、2 和 5 转化为 CO 2,将碳 3 和 4 转化为乙烯,这是一种四电子氧化,与由所有其他 Fe 介导的 2OG 脱羧为琥珀酸盐的简单脱羧截然不同/2OG酶。EFE 还催化一个次要反应,其中正常的脱羧与l-精氨酸(EF 途径所需的活化剂)的氧化相结合,导致其转化为l-谷氨酸半醛和胍。在这里,我们表明,与先例一致,l -Arg-氧化(RO)途径通过铁(IV)-氧代(ferryl)中间体进行。使用 5,5-[ 2H 2 ]- l -Arg 将 Ferrel 复合物的衰变减慢 > 16 倍,这意味着 RO 是由来自 C5 的氢原子转移 (HAT) 引发的。这种大底物氘动力学同位素效应对 EF:RO 分配比没有影响,这意味着相同的 Ferrel 中间体不能出现在 EF 通路上;途径必须更早地分道扬镳。与该结论一致,带有 Asp191Glu 配体取代的变体酶积累的 Ferrel 复合物是野生型酶的 4 倍,EF:RO 分配比降低了 40 倍。这种对 EF 途径的近乎保守的替代的选择性损害意味着它具有异常严格的立体电子要求。一个活性位点,同电荷胍对,涉及l -Arg 底物/活化剂是 EFE 在四种晶体学特征的l -Arg 修饰 Fe/2OG 加氧酶中所独有的,可用于选择性地稳定导致独特 EF 分支的过渡态。

































 京公网安备 11010802027423号
京公网安备 11010802027423号