当前位置:
X-MOL 学术
›
ACS Appl. Nano Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effects of Different Copper Species on the Combustion of Dichloromethane over Cu/HZSM-5 Zeolite Nanoporous Catalysts
ACS Applied Nano Materials ( IF 5.3 ) Pub Date : 2021-01-28 , DOI: 10.1021/acsanm.0c03156 Yue Liu 1 , Yanye Zhu 1 , Wenzuo Huang 1 , Qingji Ying 1 , Zhongbiao Wu 1, 2
ACS Applied Nano Materials ( IF 5.3 ) Pub Date : 2021-01-28 , DOI: 10.1021/acsanm.0c03156 Yue Liu 1 , Yanye Zhu 1 , Wenzuo Huang 1 , Qingji Ying 1 , Zhongbiao Wu 1, 2
Affiliation
|
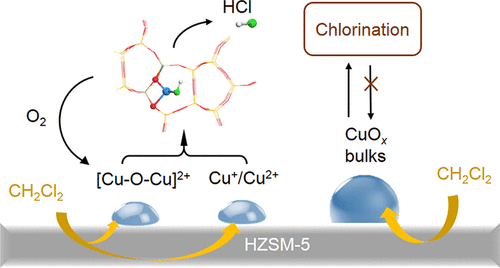
This study compared the reaction behaviors of dichloromethane (DCM) catalytic combustion over Cu/HZSM-5 (H form of Zeolite Socony Mobil-5) nanoporous catalysts at different copper loadings. Among the samples investigated, Cu/HZSM-5 with 2.5 wt % Cu showed the best stability with a 90% DCM conversion at 320 °C during a 3 h reaction run. The analysis of the results showed different copper species (including cations and oxides particles) accounted for the diverse reaction behaviors observed. With a low copper loading (≤2.5 wt %), copper cations (such as isolated Cu+/Cu2+ and oxocations) were the dominant copper species, which were strongly resistant to chlorine poisoning. However, we found the continuous coking deactivation of Cu/HZSM-5 with 1 wt % Cu loading owing to its low redox capacity. With further increase in the Cu content, the proportion of bulk-like CuOx species quickly increased. During the reaction with DCM, CuOx particles were involved in a chlorination process at a bulk level with the formation of CuClx and Cux(OH)yCl crystalline phases, resulting in a rapid deactivation at the initial reaction stage. Cation-dominant copper nanomaterials are recommended for reaching a high stability in chlorinated volatile organic compound catalytic oxidation.
中文翻译:

Cu / HZSM-5沸石纳米孔催化剂上不同铜形态对二氯甲烷燃烧的影响
这项研究比较了在不同铜负载下,二氯甲烷(DCM)在Cu / HZSM-5(H型沸石Socony Mobil-5的H形式)纳米多孔催化剂上的催化燃烧反应行为。在所研究的样品中,Cu / HZSM-5和Cu的含量为2.5 wt%,在3小时的反应过程中,在320°C时,DCM转化率为90%,显示出最佳的稳定性。结果分析表明,不同的铜种类(包括阳离子和氧化物颗粒)说明了观察到的各种反应行为。铜负载量低(≤2.5wt%)时,铜阳离子(如孤立的Cu + / Cu 2+和含氧化合物)是占主导地位的铜,对氯中毒具有很强的抵抗力。然而,由于其低氧化还原容量,我们发现具有1wt%的铜负载量的Cu / HZSM-5连续焦化失活。随着Cu含量的进一步增加,块状CuO x物种的比例迅速增加。在与DCM反应期间,CuO x颗粒以整体水平参与氯化过程,形成CuCl x和Cu x(OH)y Cl结晶相,导致在初始反应阶段迅速失活。推荐使用阳离子为主的铜纳米材料,以便在氯化挥发性有机化合物的催化氧化中达到较高的稳定性。
更新日期:2021-02-26
中文翻译:

Cu / HZSM-5沸石纳米孔催化剂上不同铜形态对二氯甲烷燃烧的影响
这项研究比较了在不同铜负载下,二氯甲烷(DCM)在Cu / HZSM-5(H型沸石Socony Mobil-5的H形式)纳米多孔催化剂上的催化燃烧反应行为。在所研究的样品中,Cu / HZSM-5和Cu的含量为2.5 wt%,在3小时的反应过程中,在320°C时,DCM转化率为90%,显示出最佳的稳定性。结果分析表明,不同的铜种类(包括阳离子和氧化物颗粒)说明了观察到的各种反应行为。铜负载量低(≤2.5wt%)时,铜阳离子(如孤立的Cu + / Cu 2+和含氧化合物)是占主导地位的铜,对氯中毒具有很强的抵抗力。然而,由于其低氧化还原容量,我们发现具有1wt%的铜负载量的Cu / HZSM-5连续焦化失活。随着Cu含量的进一步增加,块状CuO x物种的比例迅速增加。在与DCM反应期间,CuO x颗粒以整体水平参与氯化过程,形成CuCl x和Cu x(OH)y Cl结晶相,导致在初始反应阶段迅速失活。推荐使用阳离子为主的铜纳米材料,以便在氯化挥发性有机化合物的催化氧化中达到较高的稳定性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号