当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fragment-Based Discovery of Novel Allosteric MEK1 Binders
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2021-01-27 , DOI: 10.1021/acsmedchemlett.0c00563
Paolo Di Fruscia 1 , Fredrik Edfeldt 2 , Igor Shamovsky 3 , Gavin W Collie 1 , Anna Aagaard 2 , Louise Barlind 4 , Ulf Börjesson 2 , Eva L Hansson 5 , Richard J Lewis 3 , Magnus K Nilsson 3 , Linda Öster 2 , Josefine Pemberton 2 , Lena Ripa 3 , R Ian Storer 1 , Helena Käck 2
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2021-01-27 , DOI: 10.1021/acsmedchemlett.0c00563
Paolo Di Fruscia 1 , Fredrik Edfeldt 2 , Igor Shamovsky 3 , Gavin W Collie 1 , Anna Aagaard 2 , Louise Barlind 4 , Ulf Börjesson 2 , Eva L Hansson 5 , Richard J Lewis 3 , Magnus K Nilsson 3 , Linda Öster 2 , Josefine Pemberton 2 , Lena Ripa 3 , R Ian Storer 1 , Helena Käck 2
Affiliation
|
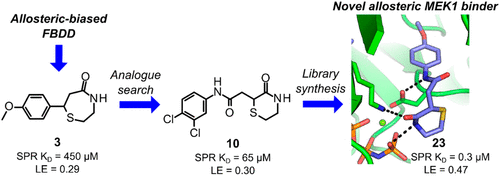
The MEK1 kinase plays a critical role in key cellular processes, and as such, its dysfunction is strongly linked to several human diseases, particularly cancer. MEK1 has consequently received considerable attention as a drug target, and a significant number of small-molecule inhibitors of this kinase have been reported. The majority of these inhibitors target an allosteric pocket proximal to the ATP binding site which has proven to be highly druggable, with four allosteric MEK1 inhibitors approved to date. Despite the significant attention that the MEK1 allosteric site has received, chemotypes which have been shown structurally to bind to this site are limited. With the aim of discovering novel allosteric MEK1 inhibitors using a fragment-based approach, we report here a screening method which resulted in the discovery of multiple allosteric MEK1 binders, one series of which was optimized to sub-μM affinity for MEK1 with promising physicochemical and ADMET properties.
中文翻译:

基于片段的新型变构 MEK1 结合物的发现
MEK1 激酶在关键细胞过程中发挥着关键作用,因此,其功能障碍与多种人类疾病,特别是癌症密切相关。因此,MEK1 作为药物靶点受到了相当多的关注,并且已经报道了大量该激酶的小分子抑制剂。这些抑制剂中的大多数靶向靠近 ATP 结合位点的变构袋,该变构袋已被证明具有高度的成药性,迄今为止有四种变构 MEK1 抑制剂已获批准。尽管 MEK1 变构位点受到了极大的关注,但在结构上显示与该位点结合的化学型仍然有限。为了使用基于片段的方法发现新型变构 MEK1 抑制剂,我们在此报告了一种筛选方法,该方法发现了多种变构 MEK1 结合剂,其中一系列经过优化,对 MEK1 的亲和力达到亚μM,具有良好的物理化学和ADMET 属性。
更新日期:2021-02-11
中文翻译:

基于片段的新型变构 MEK1 结合物的发现
MEK1 激酶在关键细胞过程中发挥着关键作用,因此,其功能障碍与多种人类疾病,特别是癌症密切相关。因此,MEK1 作为药物靶点受到了相当多的关注,并且已经报道了大量该激酶的小分子抑制剂。这些抑制剂中的大多数靶向靠近 ATP 结合位点的变构袋,该变构袋已被证明具有高度的成药性,迄今为止有四种变构 MEK1 抑制剂已获批准。尽管 MEK1 变构位点受到了极大的关注,但在结构上显示与该位点结合的化学型仍然有限。为了使用基于片段的方法发现新型变构 MEK1 抑制剂,我们在此报告了一种筛选方法,该方法发现了多种变构 MEK1 结合剂,其中一系列经过优化,对 MEK1 的亲和力达到亚μM,具有良好的物理化学和ADMET 属性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号