当前位置:
X-MOL 学术
›
J. Cell. Physiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CYLD mediates human pulmonary artery smooth muscle cell dysfunction in congenital heart disease-associated pulmonary arterial hypertension
Journal of Cellular Physiology ( IF 4.5 ) Pub Date : 2021-01-28 , DOI: 10.1002/jcp.30298 Jing-Jing Zhou 1 , Huang Li 2 , Li Li 3 , Yue Li 4 , Pei-He Wang 4 , Xian-Min Meng 5 , Jian-Guo He 1
Journal of Cellular Physiology ( IF 4.5 ) Pub Date : 2021-01-28 , DOI: 10.1002/jcp.30298 Jing-Jing Zhou 1 , Huang Li 2 , Li Li 3 , Yue Li 4 , Pei-He Wang 4 , Xian-Min Meng 5 , Jian-Guo He 1
Affiliation
|
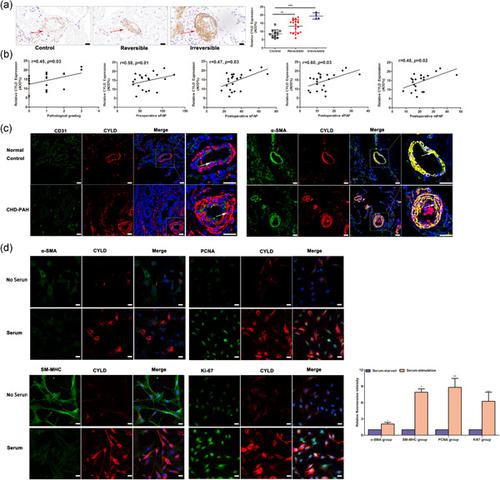
Pulmonary arterial hypertension (PAH) is a common complication of congenital heart disease (CHD). Deubiquitinase cylindromatosis (CYLD) has been reported to significantly aggravate vascular smooth muscle cell (VSMC) phenotypic transformation, proliferation, and migration. Here, we aimed to further investigate its roles and underlying mechanisms in the CHD-PAH development. The expression of CYLD in the lung tissues from CHD-PAH patients and monocrotaline (MCT) plus aortocaval (AV)-induced PAH rats, pulmonary artery smooth muscle cells (PASMCs) from MCT-AV-induced PAH rats, and human PASMCs (HPASMCs) was evaluated. After infection with CYLD siRNA or pcNDA3.1-CYLD, the proliferation, migration, and apoptosis of HPASMCs were measured using an EdU assay, transwell and scratch wound healing assays, and flow cytometric assay, respectively. An adeno-associated virus (AAV) vector encoding CYLD was used to suppress CYLD expression by being intratracheally instilled in rats 7 days before MCT-AV treatment. The results showed that CYLD was increased in the lung tissues from CHD-PAH patients and MCT-AV-induced PAH rats, and in PASMCs from MCT-AV-induced PAH rats. The contractile-type HPASMCs expressed low levels of CYLD, while the proliferative synthetic-type HPASMCs expressed high levels of CYLD. In addition, CYLD could mediate HPASMC dysfunction, which regulated HPASMC phenotypic transformation and proliferation via the modulation of p38 and ERK activation, while CYLD regulated HPASMC migration via the modulation of p38 activation. In vivo results demonstrated that the local suppression of CYLD expression could attenuate the increased levels of PAH and its associated pulmonary vascular remodeling in MCT-AV-induced PAH rats. Collectively, these results indicated that CYLD might be a potential novel therapeutic target for the prevention of PAH and pulmonary vascular remodeling in CHD-PAH through the modulation of HPASMC dysfunction.
中文翻译:

CYLD介导先天性心脏病相关肺动脉高压中的人肺动脉平滑肌细胞功能障碍
肺动脉高压(PAH)是先天性心脏病(CHD)的常见并发症。据报道,去泛素酶圆柱瘤病 (CYLD) 会显着加重血管平滑肌细胞 (VSMC) 表型转化、增殖和迁移。在这里,我们的目的是进一步研究其在 CHD-PAH 发展中的作用和潜在机制。 CHD-PAH患者和野百合碱(MCT)加主动脉腔静脉(AV)诱导的PAH大鼠肺组织、MCT-AV诱导的PAH大鼠肺动脉平滑肌细胞(PASMC)和人PASMC(HPASMC)中CYLD的表达)进行了评价。用CYLD siRNA或pcNDA3.1-CYLD感染后,分别使用EdU测定、transwell和划痕伤口愈合测定以及流式细胞术测定HPASMC的增殖、迁移和凋亡。在MCT-AV治疗前7天,通过将编码CYLD的腺相关病毒(AAV)载体注入大鼠气管内来抑制CYLD表达。结果显示,CHD-PAH 患者和 MCT-AV 诱导的 PAH 大鼠的肺组织以及 MCT-AV 诱导的 PAH 大鼠的 PASMC 中 CYLD 增加。收缩型HPASMCs表达低水平的CYLD,而增殖合成型HPASMCs表达高水平的CYLD。此外,CYLD可以介导HPASMC功能障碍,通过调节p38和ERK激活来调节HPASMC表型转化和增殖,而CYLD通过调节p38激活来调节HPASMC迁移。体内结果表明,CYLD 表达的局部抑制可以减弱 MCT-AV 诱导的 PAH 大鼠中 PAH 水平的升高及其相关的肺血管重塑。 总的来说,这些结果表明 CYLD 可能是通过调节 HPASMC 功能障碍来预防 PAH 和 CHD-PAH 肺血管重塑的潜在新治疗靶点。
更新日期:2021-01-28
中文翻译:

CYLD介导先天性心脏病相关肺动脉高压中的人肺动脉平滑肌细胞功能障碍
肺动脉高压(PAH)是先天性心脏病(CHD)的常见并发症。据报道,去泛素酶圆柱瘤病 (CYLD) 会显着加重血管平滑肌细胞 (VSMC) 表型转化、增殖和迁移。在这里,我们的目的是进一步研究其在 CHD-PAH 发展中的作用和潜在机制。 CHD-PAH患者和野百合碱(MCT)加主动脉腔静脉(AV)诱导的PAH大鼠肺组织、MCT-AV诱导的PAH大鼠肺动脉平滑肌细胞(PASMC)和人PASMC(HPASMC)中CYLD的表达)进行了评价。用CYLD siRNA或pcNDA3.1-CYLD感染后,分别使用EdU测定、transwell和划痕伤口愈合测定以及流式细胞术测定HPASMC的增殖、迁移和凋亡。在MCT-AV治疗前7天,通过将编码CYLD的腺相关病毒(AAV)载体注入大鼠气管内来抑制CYLD表达。结果显示,CHD-PAH 患者和 MCT-AV 诱导的 PAH 大鼠的肺组织以及 MCT-AV 诱导的 PAH 大鼠的 PASMC 中 CYLD 增加。收缩型HPASMCs表达低水平的CYLD,而增殖合成型HPASMCs表达高水平的CYLD。此外,CYLD可以介导HPASMC功能障碍,通过调节p38和ERK激活来调节HPASMC表型转化和增殖,而CYLD通过调节p38激活来调节HPASMC迁移。体内结果表明,CYLD 表达的局部抑制可以减弱 MCT-AV 诱导的 PAH 大鼠中 PAH 水平的升高及其相关的肺血管重塑。 总的来说,这些结果表明 CYLD 可能是通过调节 HPASMC 功能障碍来预防 PAH 和 CHD-PAH 肺血管重塑的潜在新治疗靶点。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号