当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium-Catalyzed Diastereo- and Enantioselective [3 + 2] Cycloaddition of Vinylcyclopropanes with Azadienes: Efficient Access to Chiral Spirocycles
Organic Letters ( IF 4.9 ) Pub Date : 2021-01-27 , DOI: 10.1021/acs.orglett.0c04062
Kai Liu 1 , Jianfeng Yang 1 , Xiaoxun Li 1, 2
Organic Letters ( IF 4.9 ) Pub Date : 2021-01-27 , DOI: 10.1021/acs.orglett.0c04062
Kai Liu 1 , Jianfeng Yang 1 , Xiaoxun Li 1, 2
Affiliation
|
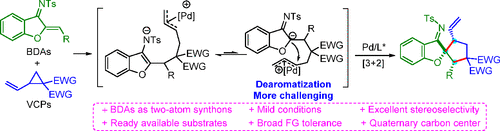
Benzofuran-derived azadienes (BDAs) have been widely used as four-atom synthons in transition-metal-mediated cycloaddition reactions, while the exploitation of their reactivity as a two-atom unit to construct spirocycles is still underdeveloped. Herein, we reported the first palladium(0)-catalyzed diastereo- and enantioselective [3 + 2] annulation of vinylcyclopropanes (VCPs) and BDAs. This transformation is featured with a broad substrate scope (31 examples), allowing for facile access to a variety of enantioenriched spirocycles bearing a quaternary stereogenic center in good yields with excellent regio-, diastereo-, and enantioselectivities (up to 93% yield, >20:1 dr, and mostly >99% ee) under mild reaction conditions. Moreover, the spirocyclic products could be efficiently converted to structurally complex tricyclo[8.3.0.01,5]-azatridecane and tricyclo[7.3.0.01,5]-azadodecane skeletons.
中文翻译:

钯催化的乙烯基环丙烷与氮杂二烯的非对映和对映选择性[3 + 2]环加成反应:有效地获得手性螺环
苯并呋喃衍生的氮杂二烯(BDA)已广泛用作过渡金属介导的环加成反应中的四原子合成子,而利用其作为二原子单元构建螺环的反应性仍然不发达。在这里,我们报道了乙烯基环丙烷(VCP)和BDA的第一个钯(0)催化的非对映和对映选择性[3 + 2]环化反应。该转化具有广泛的底物范围(31个实例),可轻松获得具有季立体位中心的多种对映体富集的螺环,产率高,具有良好的区域,非对映和对映选择性(高达93%的产率,>在温和的反应条件下为20:1 dr,并且大多数> 99%ee)。而且,螺环产物可以有效地转化为结构复杂的三环[8.3.0.01,5 ]-氮杂十七烷和三环[7.3.0.0 1,5 ]-氮杂十二烷骨架。
更新日期:2021-02-05
中文翻译:

钯催化的乙烯基环丙烷与氮杂二烯的非对映和对映选择性[3 + 2]环加成反应:有效地获得手性螺环
苯并呋喃衍生的氮杂二烯(BDA)已广泛用作过渡金属介导的环加成反应中的四原子合成子,而利用其作为二原子单元构建螺环的反应性仍然不发达。在这里,我们报道了乙烯基环丙烷(VCP)和BDA的第一个钯(0)催化的非对映和对映选择性[3 + 2]环化反应。该转化具有广泛的底物范围(31个实例),可轻松获得具有季立体位中心的多种对映体富集的螺环,产率高,具有良好的区域,非对映和对映选择性(高达93%的产率,>在温和的反应条件下为20:1 dr,并且大多数> 99%ee)。而且,螺环产物可以有效地转化为结构复杂的三环[8.3.0.01,5 ]-氮杂十七烷和三环[7.3.0.0 1,5 ]-氮杂十二烷骨架。































 京公网安备 11010802027423号
京公网安备 11010802027423号