当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Antifouling Dendrimer-Entrapped Copper Sulfide Nanoparticles Enable Photoacoustic Imaging-Guided Targeted Combination Therapy of Tumors and Tumor Metastasis
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-01-27 , DOI: 10.1021/acsami.0c21620
Zhijun Ouyang 1 , Du Li 1 , Zhijuan Xiong 1 , Cong Song 1 , Yue Gao 1 , Renna Liu 1 , Mingwu Shen 1 , Xiangyang Shi 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-01-27 , DOI: 10.1021/acsami.0c21620
Zhijun Ouyang 1 , Du Li 1 , Zhijuan Xiong 1 , Cong Song 1 , Yue Gao 1 , Renna Liu 1 , Mingwu Shen 1 , Xiangyang Shi 1
Affiliation
|
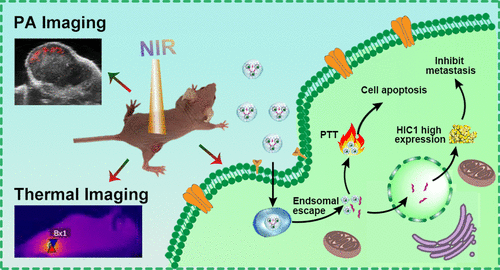
The development of functional intelligent theranostic nanoplatform for imaging-directed synchronous inhibition of primary tumor and tumor metastasis is still a challenging task. We present here the creation of functional dendrimer-entrapped CuS nanoparticles (CuS DENPs) complexed with plasmid DNA-encoding hypermethylation in cancer 1 (pDNA-HIC1) for photoacoustic (PA) imaging-directed simultaneous inhibition of tumors and tumor metastasis. Poly(amidoamine) dendrimers of generation 5 were covalently attached with 1,3-propane sultone and arginine-glycine-aspartic acid (RGD) peptide through a spacer of poly(ethylene glycol) and adopted for the templated synthesis of CuS NPs. The prepared functional RGD-CuS DENPs possess a mean CuS core diameter of 4.2 nm, good colloidal stability, and an excellent absorption feature in the second near-infrared window, thus having a photothermal conversion efficiency of 49.8% and an outstanding PA imaging capability. The functional DENPs can effectively deliver pDNA-HIC1 to prevent cancer cell invasion and metastasis in a serum-enhancing manner by virtue of zwitterionic modification-rendered antifouling property. The developed RGD-CuS DENPs/pDNA polyplexes display αvβ3 integrin-targeted enhanced anticancer activity through the combined CuS NP-mediated photothermal therapy (PTT) and pDNA delivery-rendered cancer cell metastasis inhibition. This can also be proven by the therapeutic efficacy of a triple-negative breast cancer model in vivo, where inhibition of both the primary subcutaneous tumor and lung metastasis can be realized. The created dendrimer-CuS hybrid nanoplatform represents one of the updated designs of nanomedicine for PA imaging-directed combination PTT/gene therapy of tumors and tumor metastasis.
中文翻译:

防污树枝状大分子包裹的硫化铜纳米粒子能够实现光声成像引导的靶向靶向治疗肿瘤和肿瘤转移的联合治疗。
用于成像指导的同步抑制原发肿瘤和肿瘤转移的功能性智能治疗药物纳米平台的开发仍然是一项艰巨的任务。我们在这里介绍功能性树状大分子包裹的CuS纳米粒子(CuS DENPs)的创建与质粒1编码超甲基化在癌症1(pDNA-HIC1)中的光声(PA)成像定向同时抑制肿瘤和肿瘤转移。通过聚乙二醇的间隔基将第5代的聚(酰胺基胺)树状聚合物与1,3-丙烷磺酸内酯和精氨酸-甘氨酸-天冬氨酸(RGD)肽共价连接,并用于CuS NP的模板合成。制备的功能性RGD-CuS DENP的平均CuS核心直径为4.2 nm,具有良好的胶体稳定性,并且在第二个近红外窗口中具有出色的吸收特性,因此具有49.8%的光热转换效率和出色的PA成像能力。借助两性离子修饰赋予的防污性能,功能性DENP可以以增强血清的方式有效递送pDNA-HIC1,以防止癌细胞的侵袭和转移。已开发的RGD-CuS DENPs / pDNA多聚体显示αv β 3整联蛋白-靶向增强通过组合的CuS NP介导的光热治疗(PTT)和pDNA的递送渲染癌细胞转移抑制的抗癌活性。这也可以通过体内三阴性乳腺癌模型的治疗效果来证明,其中可以实现对原发性皮下肿瘤和肺转移的抑制。创建的树状聚合物-CuS杂化纳米平台代表了用于PA成像指导的肿瘤和肿瘤转移基因联合治疗的纳米药物的更新设计之一。
更新日期:2021-02-10
中文翻译:

防污树枝状大分子包裹的硫化铜纳米粒子能够实现光声成像引导的靶向靶向治疗肿瘤和肿瘤转移的联合治疗。
用于成像指导的同步抑制原发肿瘤和肿瘤转移的功能性智能治疗药物纳米平台的开发仍然是一项艰巨的任务。我们在这里介绍功能性树状大分子包裹的CuS纳米粒子(CuS DENPs)的创建与质粒1编码超甲基化在癌症1(pDNA-HIC1)中的光声(PA)成像定向同时抑制肿瘤和肿瘤转移。通过聚乙二醇的间隔基将第5代的聚(酰胺基胺)树状聚合物与1,3-丙烷磺酸内酯和精氨酸-甘氨酸-天冬氨酸(RGD)肽共价连接,并用于CuS NP的模板合成。制备的功能性RGD-CuS DENP的平均CuS核心直径为4.2 nm,具有良好的胶体稳定性,并且在第二个近红外窗口中具有出色的吸收特性,因此具有49.8%的光热转换效率和出色的PA成像能力。借助两性离子修饰赋予的防污性能,功能性DENP可以以增强血清的方式有效递送pDNA-HIC1,以防止癌细胞的侵袭和转移。已开发的RGD-CuS DENPs / pDNA多聚体显示αv β 3整联蛋白-靶向增强通过组合的CuS NP介导的光热治疗(PTT)和pDNA的递送渲染癌细胞转移抑制的抗癌活性。这也可以通过体内三阴性乳腺癌模型的治疗效果来证明,其中可以实现对原发性皮下肿瘤和肺转移的抑制。创建的树状聚合物-CuS杂化纳米平台代表了用于PA成像指导的肿瘤和肿瘤转移基因联合治疗的纳米药物的更新设计之一。

































 京公网安备 11010802027423号
京公网安备 11010802027423号