Journal of Colloid and Interface Science ( IF 9.4 ) Pub Date : 2021-01-27 , DOI: 10.1016/j.jcis.2021.01.061
Patrick Denk , Asmae El Maangar , Jyotsana Lal , David Kleber , Thomas Zemb , Werner Kunz
|
Hypothesis
The surfactant C8EO8CH2COOH (Akypo LF2) and its salts have a small hydrophobic and a significantly longer hydrophilic part. As a consequence, there must be a significant steric constraint, once these surfactant molecules form micelles. In addition, the partially charged headgroups should bring some additional fine-tuning via electrostatic interactions to this “essentially non-ionic” surfactant.
Experiments
Phase diagrams of binary mixtures of water and C8EO8CH2COOH are established over large concentration and temperature ranges, also at different pHs and in the presence of sodium and calcium ions. Surface tensions and osmotic pressures are measured to understand the systems. To evaluate the microstructures, also Dynamic Light Scattering and Small-Angle X-ray Scattering are performed.
Findings
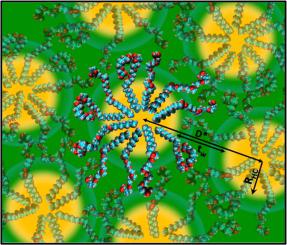
Apart from the formation of coacervates at very low surfactant concentrations, spherical micelles persist over the whole concentration and temperature range and do not change in size and shape. At very high surfactant concentrations, above 60% by weight, where the headgroups are no longer fully hydrated, the standard core-shell structure of micelles vanishes and highly stabilized aggregates of 8–26 octyl chains are suspended in interdigitated polyoxyethylene layers and form an “osmotic brush”. When the acid is partially transformed to a sodium salt, the repulsion between the micelles increases, whereas bridging between micelles prevails, when the counterions are calcium cations. Remarkably, the negative charges of the headgroups are randomly distributed in the hydrophilic ethylene oxide shell. Altogether, a phase diagram without lyotropic liquid crystalline phases and an extreme shift of the cloud-point in temperature and composition is found, similar to the phase diagram of C8EO8OH already known in literature. The phase properties can be explained by the curvature and packing constraints together with the Lindemann rule applied to short hydrocarbon chains.
中文翻译:

烷基短链聚乙二醇醚羧酸盐和羧酸三嵌段表面活性剂水溶液的相图和微观结构
假设
表面活性剂C 8 EO 8 CH 2 COOH(Akypo LF2)及其盐具有小的疏水性和明显更长的亲水性部分。结果,一旦这些表面活性剂分子形成胶束,就必须存在明显的空间约束。此外,部分带电的头基应该通过静电相互作用为这种“基本上非离子的”表面活性剂带来一些额外的微调。
实验
水和C 8 EO 8 CH 2 COOH的二元混合物的相图是在较大的浓度和温度范围内,在不同的pH值以及在钠和钙离子存在的情况下建立的。测量表面张力和渗透压以了解系统。为了评估微观结构,还进行了动态光散射和小角度X射线散射。
发现
除了在非常低的表面活性剂浓度下形成凝聚层以外,球形胶束在整个浓度和温度范围内均会持续存在,并且尺寸和形状不会改变。在非常高的表面活性剂浓度(高于60%重量)时,头基不再完全水合,胶束的标准核-壳结构消失,高度稳定的8-26个辛基链聚集体悬浮在指状聚氧乙烯层中,形成“渗透刷”。当酸部分转化为钠盐时,当抗衡离子为钙阳离子时,胶束之间的排斥力增加,而胶束之间的桥接占优势。显着地,头基的负电荷随机分布在亲水环氧乙烷壳中。共,8 EO 8 OH在文献中已经知道。相特性可以通过曲率和堆积约束以及应用于短烃链的林德曼法则来解释。































 京公网安备 11010802027423号
京公网安备 11010802027423号