当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hexadehydro-Diels–Alder Reaction: Benzyne Generation via Cycloisomerization of Tethered Triynes
Chemical Reviews ( IF 51.4 ) Pub Date : 2021-01-25 , DOI: 10.1021/acs.chemrev.0c00825
Lucas L Fluegel 1 , Thomas R Hoye 1
Chemical Reviews ( IF 51.4 ) Pub Date : 2021-01-25 , DOI: 10.1021/acs.chemrev.0c00825
Lucas L Fluegel 1 , Thomas R Hoye 1
Affiliation
|
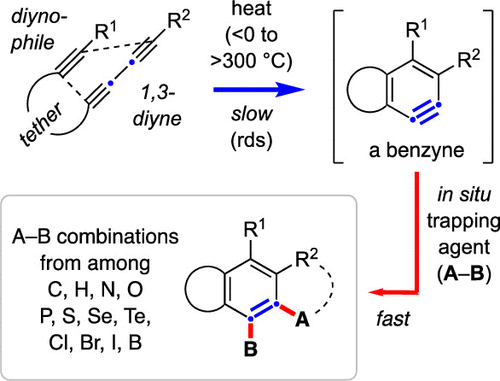
The hexadehydro-Diels–Alder (HDDA) reaction is the thermal cyclization of an alkyne and a 1,3-diyne to generate a benzyne intermediate. This is then rapidly trapped, in situ, by a variety of species to yield highly functionalized benzenoid products. In contrast to nearly all other methods of aryne generation, no other reagents are required to produce an HDDA benzyne. The versatile and customizable nature of the process has attracted much attention due not only to its synthetic potential but also because of the fundamental mechanistic insights the studies often afford. The authors have attempted to provide here a comprehensive compilation of publications appearing by mid-2020 that describe experimental results of HDDA reactions.
中文翻译:

六氢狄尔斯-阿尔德反应:通过束缚三炔的环异构化生成苯炔
十六氢-狄尔斯-阿尔德 (HDDA) 反应是炔烃和 1,3-二炔的热环化,生成苯炔中间体。然后,它被多种物质快速原位捕获,产生高度功能化的苯类产品。与几乎所有其他芳炔生成方法相比,不需要其他试剂来生产 HDDA 苯炔。该过程的多功能性和可定制性引起了广泛关注,不仅因为它的合成潜力,还因为这些研究经常提供的基本机制见解。作者试图在此提供 2020 年中期发表的出版物的综合汇编,描述 HDDA 反应的实验结果。
更新日期:2021-02-24
中文翻译:

六氢狄尔斯-阿尔德反应:通过束缚三炔的环异构化生成苯炔
十六氢-狄尔斯-阿尔德 (HDDA) 反应是炔烃和 1,3-二炔的热环化,生成苯炔中间体。然后,它被多种物质快速原位捕获,产生高度功能化的苯类产品。与几乎所有其他芳炔生成方法相比,不需要其他试剂来生产 HDDA 苯炔。该过程的多功能性和可定制性引起了广泛关注,不仅因为它的合成潜力,还因为这些研究经常提供的基本机制见解。作者试图在此提供 2020 年中期发表的出版物的综合汇编,描述 HDDA 反应的实验结果。

































 京公网安备 11010802027423号
京公网安备 11010802027423号