当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bioinspired Redox Mediator in Lithium–Oxygen Batteries
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-01-26 , DOI: 10.1021/acscatal.0c04544 Muqing Ren 1 , Jinhang Chen 1 , Gang Wu 2 , Emily A. McHugh 1 , Ah-Lim Tsai 2 , James M. Tour 1, 3, 4
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-01-26 , DOI: 10.1021/acscatal.0c04544 Muqing Ren 1 , Jinhang Chen 1 , Gang Wu 2 , Emily A. McHugh 1 , Ah-Lim Tsai 2 , James M. Tour 1, 3, 4
Affiliation
|
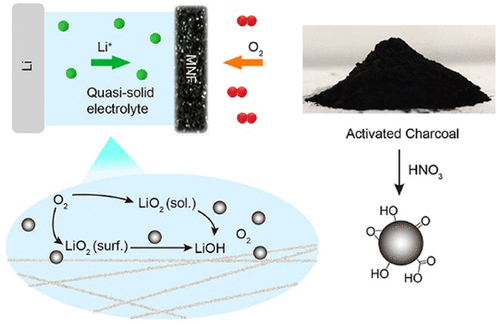
LiO2 is an intermediate formed in aprotic Li–O2 and Li–air batteries during an oxygen reduction reaction (ORR). The soluble LiO2 triggers undesirable side reactions, such as a nucleophilic reaction and the formation of the byproduct Li2CO3 upon reaction with the electrolyte and substrate, resulting in irreversible redox reactions and causing a low Coulombic efficiency with poor cycling durability. Inspired by superoxide dismutase (SOD) in biological systems, we hypothesized that SOD mimetics could likewise be applied in Li–O2 and Li–air batteries, thereby prolonging the battery cycle life. Oxidized activated charcoal (OAC) is known to be one SOD mimetic with fast kinetics and a high turnover. Therefore, OAC was added to a dual polymer gel electrolyte as a redox mediator. The OAC redox mechanism is well illustrated in quasi-solid Li–O2 batteries, and the battery stability was significantly improved in the presence of OAC. Furthermore, the best Li–O2 battery containing OAC demonstrated stable galvanostatic charge/discharge performance for ∼300 cycles (3000 h) with a cutoff capacity of 0.4 mAh cm–2. In addition, under a discharge cutoff potential of 2.0 V, the discharge capacity of the best Li–O2 battery is ∼37.0 mAh cm–2.
中文翻译:

锂氧电池中受生物启发的氧化还原介体
LiO 2是非质子型Li-O 2和Li-空气电池在氧还原反应(ORR)中形成的中间体。可溶性LiO 2引发不良的副反应,例如亲核反应以及在与电解质和底物反应时形成副产物Li 2 CO 3,导致不可逆的氧化还原反应并导致库仑效率低,循环耐久性差。受生物系统中超氧化物歧化酶(SOD)的启发,我们假设SOD模拟物同样可以应用于Li–O 2中。和锂空气电池,从而延长了电池循环寿命。氧化活性炭(OAC)是一种具有快速动力学和高周转率的SOD模拟物。因此,将OAC作为氧化还原介质添加到双聚合物凝胶电解质中。在准固态Li–O 2电池中可以很好地说明OAC的氧化还原机制,并且在存在OAC的情况下电池的稳定性得到了显着改善。此外,最好的包含OAC的Li–O 2电池在300个循环(3000小时)中表现出稳定的恒电流充电/放电性能,截止容量为0.4 mAh cm –2。另外,在2.0 V的放电截止电位下,最佳的Li–O 2电池的放电容量约为37.0 mAh cm –2。
更新日期:2021-02-05
中文翻译:

锂氧电池中受生物启发的氧化还原介体
LiO 2是非质子型Li-O 2和Li-空气电池在氧还原反应(ORR)中形成的中间体。可溶性LiO 2引发不良的副反应,例如亲核反应以及在与电解质和底物反应时形成副产物Li 2 CO 3,导致不可逆的氧化还原反应并导致库仑效率低,循环耐久性差。受生物系统中超氧化物歧化酶(SOD)的启发,我们假设SOD模拟物同样可以应用于Li–O 2中。和锂空气电池,从而延长了电池循环寿命。氧化活性炭(OAC)是一种具有快速动力学和高周转率的SOD模拟物。因此,将OAC作为氧化还原介质添加到双聚合物凝胶电解质中。在准固态Li–O 2电池中可以很好地说明OAC的氧化还原机制,并且在存在OAC的情况下电池的稳定性得到了显着改善。此外,最好的包含OAC的Li–O 2电池在300个循环(3000小时)中表现出稳定的恒电流充电/放电性能,截止容量为0.4 mAh cm –2。另外,在2.0 V的放电截止电位下,最佳的Li–O 2电池的放电容量约为37.0 mAh cm –2。

































 京公网安备 11010802027423号
京公网安备 11010802027423号