Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2021-01-26 , DOI: 10.1016/j.molliq.2021.115467 Qian Zhang , Yongzhong Jia , Jinhe Sun , Pengrui Zhang , Chaochi Huang , Mingyong Wang , Zixuan Xue , Ciming Wang , Fei Shao , Fengtai Tong , Yilin Xiao , Yan Jing
|
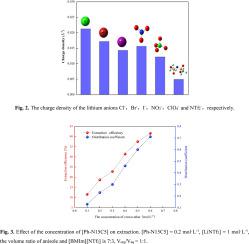
Azacrown ethers contain multiple coordination sites, which show specific complexation to alkali metal ions. In this research, a neoteric liquid-liquid extraction system was investigated for the selective separation of lithium isotope using N-phenylaza-15-crown-5 (Ph-N15C5) as chelating agent and [BMIm][NTf2] and anisole as extraction solvent. The effects of the counter anions of lithium salt, concentrations of crown ether, temperature, extraction equilibrium time and concentrations of lithium salt solution on the separation of lithium isotope with Ph-N15C5 were studied. The maximum extraction efficiency of lithium ions was 41.54% and the maximum single-stage separation factor (α) of 6Li/7Li obtained in the present study was 1.025, indicating that the lighter isotope 6Li was enriched in organic phase while the heavier isotope 7Li was concentrated in the aqueous phase. The complexation ratio of the lithium crown ether complex was determined as 1:1 by the slope analysis method. The lithium ions in the aqueous phase were extracted into the organic phase to form [Li(Ph-N15C5)]+ complex. The thermodynamic data obtained at different temperatures demonstrated that lithium isotope exchange was a spontaneous exothermic process, and lowering the temperature was beneficial to increase the separation factor. In addition, the system reached the extraction equilibrium after 60 min. This high efficiency and green liquid-liquid extraction system has potential application in lithium isotope separation.
中文翻译:

N -phenylaza-15-crown-5的锂同位素分离作用
氮杂皇冠醚包含多个配位位点,这些位点显示出与碱金属离子的特定络合。在这项研究中,研究了一种新型液-液萃取系统,以N-苯基氮杂-15-冠-5(Ph-N15C5)为螯合剂,并以[BMIm] [NTf 2 ]和苯甲醚为萃取物,选择性分离锂同位素。溶剂。研究了锂盐抗衡阴离子,冠醚浓度,温度,萃取平衡时间和锂盐溶液浓度对用Ph-N15C5分离锂同位素的影响。锂离子的最大萃取效率为41.54%,最大单级分离因子(α)为6 Li / 7在本研究中获得的Li为1.025,表明较轻的同位素6 Li富含有机相,而较重的同位素7 Li集中于水相。通过斜率分析法将锂冠醚络合物的络合比确定为1:1。将水相中的锂离子萃取到有机相中以形成[Li(Ph-N15C5)] +复杂的。在不同温度下获得的热力学数据表明,锂同位素交换是自发的放热过程,降低温度有利于增加分离因子。此外,系统在60分钟后达到萃取平衡。这种高效,绿色的液-液萃取系统在锂同位素分离中具有潜在的应用。






























 京公网安备 11010802027423号
京公网安备 11010802027423号