当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Isomeric Effect of Nano-Inhibitors on Aβ40 Fibrillation at The Nano-Bio Interface
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-01-24 , DOI: 10.1021/acsami.0c21906
Jianhang Li 1 , Guanbin Gao 1 , Xintong Tang 1 , Meng Yu 2 , Meng He 2 , Taolei Sun 2
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-01-24 , DOI: 10.1021/acsami.0c21906
Jianhang Li 1 , Guanbin Gao 1 , Xintong Tang 1 , Meng Yu 2 , Meng He 2 , Taolei Sun 2
Affiliation
|
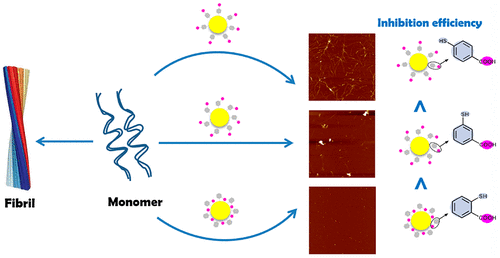
Chemical and physical properties of nanobio interface substantially affect the conformational transitions of adjacent biomolecules. Previous studies have reported the chiral effect and charge effect of nanobio interface on the misfolding, aggregation, and fibrillation of amyloid protein. However, the isomeric effect of nanobio interface on protein/peptides amyloidosis is still unclear. Here, three isomeric nanobio interfaces were designed and fabricated based on the same sized gold nanoclusters (AuNCs) modified with 4-mercaptobenzoic acid (p-MBA), 3-mercaptobenzoic acid (m-MBA), and 2-mercaptobenzoic acid (o-MBA). Then three isomeric AuNCs were employed as models to explore the isomeric effect on the misfolding, aggregation, and fibrillation of Aβ40 at nanobio interfaces. Site-specific replacement experiments on the basis of theoretical analysis revealed the possible mechanism of Aβ40 interacting with isomeric ligands of AuNCs at the nanobio interfaces. The distance and orientation of −COOH group from the surface of AuNCs can affect the electrostatic interaction between isomeric ligands and the positively charged residues (R5, K16, and K28) of Aβ40, which may affect the inhibition efficiency of isomeric AuNCs on protein amyloidosis. Actually, the amyloid fibrillation kinetics results together with atomic force microscope (AFM) images, dynamic light scattering (DLS) results and circular dichroism (CD) spectra indeed proved that all the three isomeric AuNCs could inhibit the misfolding, aggregation and fibrillation of Aβ40 in a dose-dependent manner, and the inhibition efficiency was definitely different from each other. The inhibition efficiency of o-MBA-AuNCs was higher than that of m-MBA-AuNCs and p-MBA-AuNCs at the same dosage. These results provide an insight for isomeric effect at nanobio interfaces, and open an avenue for structure-based nanodrug design target Alzheimer’s disease (AD) and even other protein conformational diseases.
中文翻译:

纳米抑制剂对纳米生物界面Aβ40纤颤的同等作用
纳米生物界面的化学和物理性质实质上影响相邻生物分子的构象转变。先前的研究已经报道了纳米生物界面对淀粉样蛋白的错误折叠,聚集和原纤化的手性作用和电荷作用。然而,纳米生物接口对蛋白质/肽淀粉样变性的异构作用仍然不清楚。在这里,三种同分异构纳米生物接口被设计和制造基于与4-巯基苯甲酸修改的同一尺寸的金纳米簇(AuNCs)(p -MBA),3-巯基苯甲酸(米-MBA),和2-巯基苯甲酸(ø - MBA)。然后以三种异构的AuNCs为模型,探讨该异构体对Aβ的错误折叠,聚集和原纤化的影响。在nanobio接口处为40。理论分析的基础上,特定场地的替代实验揭示Aβ的可能机制40与纳米生物的接口AuNCs的异构配体相互作用。从AuNCs的表面的距离和-COOH基团的取向可能会影响配体的异构体和Aβ的带正电荷的残基(R 5,K16,K28和)之间的静电相互作用40,这可能会影响AuNCs异构体对蛋白质淀粉样变性的抑制作用。实际上,用原子力显微镜淀粉样原纤化动力学的结果一起(AFM)图像,动态光散射(DLS)结果和圆二色性(CD)谱确实证明了所有三种异构体AuNCs能抑制Aβ的错误折叠,聚集和原纤化40剂量依赖性,抑制效率肯定是不同的。o -MBA-AuNCs的抑制效率高于m -MBA-AuNCs和p-MBA-AuNCs剂量相同。这些结果为了解纳米生物界面上的异构效应提供了见识,并为针对阿尔茨海默氏病(AD)甚至其他蛋白质构象性疾病的基于结构的纳米药物设计开辟了道路。
更新日期:2021-02-03
中文翻译:

纳米抑制剂对纳米生物界面Aβ40纤颤的同等作用
纳米生物界面的化学和物理性质实质上影响相邻生物分子的构象转变。先前的研究已经报道了纳米生物界面对淀粉样蛋白的错误折叠,聚集和原纤化的手性作用和电荷作用。然而,纳米生物接口对蛋白质/肽淀粉样变性的异构作用仍然不清楚。在这里,三种同分异构纳米生物接口被设计和制造基于与4-巯基苯甲酸修改的同一尺寸的金纳米簇(AuNCs)(p -MBA),3-巯基苯甲酸(米-MBA),和2-巯基苯甲酸(ø - MBA)。然后以三种异构的AuNCs为模型,探讨该异构体对Aβ的错误折叠,聚集和原纤化的影响。在nanobio接口处为40。理论分析的基础上,特定场地的替代实验揭示Aβ的可能机制40与纳米生物的接口AuNCs的异构配体相互作用。从AuNCs的表面的距离和-COOH基团的取向可能会影响配体的异构体和Aβ的带正电荷的残基(R 5,K16,K28和)之间的静电相互作用40,这可能会影响AuNCs异构体对蛋白质淀粉样变性的抑制作用。实际上,用原子力显微镜淀粉样原纤化动力学的结果一起(AFM)图像,动态光散射(DLS)结果和圆二色性(CD)谱确实证明了所有三种异构体AuNCs能抑制Aβ的错误折叠,聚集和原纤化40剂量依赖性,抑制效率肯定是不同的。o -MBA-AuNCs的抑制效率高于m -MBA-AuNCs和p-MBA-AuNCs剂量相同。这些结果为了解纳米生物界面上的异构效应提供了见识,并为针对阿尔茨海默氏病(AD)甚至其他蛋白质构象性疾病的基于结构的纳米药物设计开辟了道路。




































 京公网安备 11010802027423号
京公网安备 11010802027423号