当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, Biological Evaluation, and 3D-QSAR Studies of N-(Substituted pyridine-4-yl)-1-(substituted phenyl)-5-trifluoromethyl-1H-pyrazole-4-carboxamide Derivatives as Potential Succinate Dehydrogenase Inhibitors
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2021-01-22 , DOI: 10.1021/acs.jafc.0c05702 Zhibing Wu 1 , Hyung-Yeon Park 2 , Dewen Xie 3 , Jingxin Yang 1 , Shuaitao Hou 1 , Nasir Shahzad 2 , Chan Kyung Kim 2 , Song Yang 1
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2021-01-22 , DOI: 10.1021/acs.jafc.0c05702 Zhibing Wu 1 , Hyung-Yeon Park 2 , Dewen Xie 3 , Jingxin Yang 1 , Shuaitao Hou 1 , Nasir Shahzad 2 , Chan Kyung Kim 2 , Song Yang 1
Affiliation
|
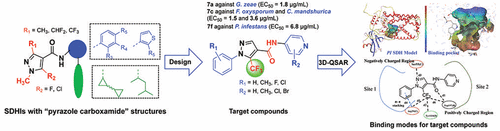
A series of new fungicides that can inhibit the succinate dehydrogenase (SDH) was classified and named as SDH inhibitors by the Fungicide Resistance Action Committee in 2009. To develop more potential SDH inhibitors, we designed and synthesized a novel series of N-(substituted pyridine-4-yl)-1-(substituted phenyl)-5-trifluoromethyl-1H-pyrazole-4-carboxamide derivatives, 4a–4i, namely, 5a–5h, 6a–6h, and 7a–7j. The bioassay results demonstrated that some title compounds exhibited excellent antifungal activity against four tested phytopathogenic fungi (Gibberella zea, Fusarium oxysporum, Cytospora mandshurica, and Phytophthora infestans). The EC50 values were 1.8 μg/mL for 7a against G. zeae, 1.5 and 3.6 μg/mL for 7c against F. oxysporum and C. mandshurica, respectively, and 6.8 μg/mL for 7f against P. infestans. The SDH enzymatic activity testing revealed that the IC50 values of 4c, 5f, 7f, and penthiopyrad were 12.5, 135.3, 6.9, and 223.9 μg/mL, respectively. The molecular docking results of this series of title compounds with SDH model demonstrated that the compounds could completely locate inside of the pocket, the body fragment formed H bonds, and the phenyl ring showed a π–π interaction with Arg59, suggesting that these novel 5-trifluoromethyl-pyrazole-4-carboxamide derivatives might target SDH. These results could provide a benchmark for understanding the antifungal activity against the phytopathogenic fungus P. infestans and prompt us to discover more potent SDH inhibitors.
中文翻译:

N-(取代的吡啶-4-基)-1-(取代的苯基)-5-三氟甲基-1H-吡唑-4-羧酰胺衍生物作为潜在的琥珀酸脱氢酶抑制剂的合成,生物学评估和3D-QSAR研究
一系列能够抑制琥珀酸脱氢酶(SDH)的新型杀真菌剂在2009年被抗真菌剂行动委员会分类并命名为SDH抑制剂。为了开发更多潜在的SDH抑制剂,我们设计并合成了一系列新型的N-(取代吡啶) -4-yl)-1-(取代的苯基)-5-三氟甲基-1 H-吡唑-4-羧酰胺衍生物4a - 4i,即5a - 5h,6a - 6h和7a - 7j。生物测定结果表明,某些标题化合物对四种受试植物病原真菌(Gibberella zea)表现出优异的抗真菌活性。,尖孢镰刀菌(Fusarium oxysporum),曼氏丝孢菌(Cytospora mandshurica)和疫霉菌(Phytophthora infestans))。的EC 50值分别为1.8微克/毫升为图7a针对G.玉蜀黍赤霉,1.5和3.6微克/毫升为7C对尖孢镰孢和C.楸分别和6.8微克/毫升1408米针对致病疫霉。SDH酶促活性测试显示IC 50值为4c,5f,7f和戊硫吡pen分别为12.5、135.3、6.9和223.9μg/ mL。该系列标题化合物具有SDH模型的分子对接结果表明,该化合物可以完全位于口袋中,体内片段形成H键,且苯环与Arg59呈π–π相互作用,表明这些新颖的5 -三氟甲基-吡唑-4-羧酰胺衍生物可能靶向SDH。这些结果可为理解抗致病性真菌致病疫霉的抗真菌活性提供基准,并促使我们发现更有效的SDH抑制剂。
更新日期:2021-02-03
中文翻译:

N-(取代的吡啶-4-基)-1-(取代的苯基)-5-三氟甲基-1H-吡唑-4-羧酰胺衍生物作为潜在的琥珀酸脱氢酶抑制剂的合成,生物学评估和3D-QSAR研究
一系列能够抑制琥珀酸脱氢酶(SDH)的新型杀真菌剂在2009年被抗真菌剂行动委员会分类并命名为SDH抑制剂。为了开发更多潜在的SDH抑制剂,我们设计并合成了一系列新型的N-(取代吡啶) -4-yl)-1-(取代的苯基)-5-三氟甲基-1 H-吡唑-4-羧酰胺衍生物4a - 4i,即5a - 5h,6a - 6h和7a - 7j。生物测定结果表明,某些标题化合物对四种受试植物病原真菌(Gibberella zea)表现出优异的抗真菌活性。,尖孢镰刀菌(Fusarium oxysporum),曼氏丝孢菌(Cytospora mandshurica)和疫霉菌(Phytophthora infestans))。的EC 50值分别为1.8微克/毫升为图7a针对G.玉蜀黍赤霉,1.5和3.6微克/毫升为7C对尖孢镰孢和C.楸分别和6.8微克/毫升1408米针对致病疫霉。SDH酶促活性测试显示IC 50值为4c,5f,7f和戊硫吡pen分别为12.5、135.3、6.9和223.9μg/ mL。该系列标题化合物具有SDH模型的分子对接结果表明,该化合物可以完全位于口袋中,体内片段形成H键,且苯环与Arg59呈π–π相互作用,表明这些新颖的5 -三氟甲基-吡唑-4-羧酰胺衍生物可能靶向SDH。这些结果可为理解抗致病性真菌致病疫霉的抗真菌活性提供基准,并促使我们发现更有效的SDH抑制剂。






























 京公网安备 11010802027423号
京公网安备 11010802027423号