当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydrodechlorination of Dichloromethane by a Metal‐Free Triazole‐Porphyrin Electrocatalyst: Demonstration of Main‐Group Element Electrocatalysis**
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2021-01-21 , DOI: 10.1002/chem.202005012 Caroline Williams 1 , Amir Lashgari 1 , Nilakshi Devi 2 , Marcus Ang 1 , Ashwin Chaturvedi 2 , Pranita Dhungana 1 , Jianbing Jimmy Jiang 2
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2021-01-21 , DOI: 10.1002/chem.202005012 Caroline Williams 1 , Amir Lashgari 1 , Nilakshi Devi 2 , Marcus Ang 1 , Ashwin Chaturvedi 2 , Pranita Dhungana 1 , Jianbing Jimmy Jiang 2
Affiliation
|
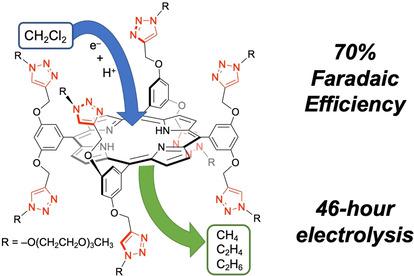
In this work, the electrocatalytic reduction of dichloromethane (CH2Cl2) into hydrocarbons involving a main group element‐based molecular triazole‐porphyrin electrocatalyst H2PorT8 is reported. This catalyst converted CH2Cl2 in acetonitrile to various hydrocarbons (methane, ethane, and ethylene) with a Faradaic efficiency of 70 % and current density of −13 mA cm−2 at a potential of −2.2 V vs. Fc/Fc+ using water as a proton source. The findings of this study and its mechanistic interpretations demonstrated that H2PorT8 was an efficient and stable catalyst for the hydrodechlorination of CH2Cl2 and that main group catalysts could be potentially used for exploring new catalytic reaction mechanisms.
中文翻译:

无金属三唑卟啉电催化剂对二氯甲烷进行加氢脱氯:主族元素电催化作用的证明**
在这项工作中,据报道,涉及一种基于主族元素的分子三唑-卟啉电催化剂H2PorT8,将二氯甲烷(CH 2 Cl 2)电催化还原为烃。该催化剂将乙腈中的CH 2 Cl 2转化为各种碳氢化合物(甲烷,乙烷和乙烯),其法拉第效率为70%,电流密度为-13 mA cm -2,电势为-2.2 V(相对于Fc / Fc +)用水作为质子源 这项研究的发现及其机理解释表明,H2PorT8是CH 2 Cl 2加氢脱氯的有效和稳定的催化剂。 并且该主族催化剂可潜在地用于探索新的催化反应机理。
更新日期:2021-01-21
中文翻译:

无金属三唑卟啉电催化剂对二氯甲烷进行加氢脱氯:主族元素电催化作用的证明**
在这项工作中,据报道,涉及一种基于主族元素的分子三唑-卟啉电催化剂H2PorT8,将二氯甲烷(CH 2 Cl 2)电催化还原为烃。该催化剂将乙腈中的CH 2 Cl 2转化为各种碳氢化合物(甲烷,乙烷和乙烯),其法拉第效率为70%,电流密度为-13 mA cm -2,电势为-2.2 V(相对于Fc / Fc +)用水作为质子源 这项研究的发现及其机理解释表明,H2PorT8是CH 2 Cl 2加氢脱氯的有效和稳定的催化剂。 并且该主族催化剂可潜在地用于探索新的催化反应机理。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号