Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2021-01-21 , DOI: 10.1016/j.apcatb.2021.119914 Xinquan Zhou , Ali Jawad , Mengyi Luo , Chunguang Luo , Tingting Zhang , Huabin Wang , Jia Wang , Songlin Wang , Zhulei Chen , Zhuqi Chen
|
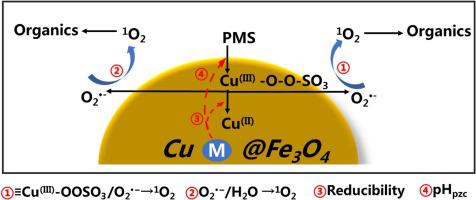
In this paper, we surprisingly found that the incorporation of unreducible metal oxides MxOy (M = Mg, Zn, Ca, Ba, Al) onto CuO hybrid magnetic nano ferric oxide (Cu@Fe3O4) may alter the reaction pathway in persulfate activation, and increase the reaction rate constant. The activation of peroxymonosulfate (PMS) by Cu@Fe3O4 led to a classic sulfate radical based oxidation process (SR-AOP) with an acetaminophen (ACE) degradation rate constant of 0.004 min−1, while 1O2-dominated nonradical oxidation process was disclosed in CuM@Fe3O4 with wildly fluctuated reaction rate constants from 0.003 to 0.242 min−1. Mechanism studies indicated that singlet oxygen (1O2) derived from the direct oxidation of superoxide anions radicals (O2 −) or the recombination of O2
−) or the recombination of O2 − was the main reactive oxygen species (ROS) in CuM@Fe3O4/PMS system. A series of characterization experiments (pHpzc tests, XPS, H2-TPR, et al.) and DFT calculation disclosed that the addition of an unreducible metal M yielded many positive effects: (1) the formation of surface oxygen vacancies (OV) raised the zero point charge (pHpzc) of CuM@Fe3O4, thus enhanced the adsorption and activation of PMS; (2) promoting the generation of a new Cu species (Cu3+) on the surface of CuM@Fe3O4, which then participated in the generation of 1O2. The different reducibility of Cu3+ led to differences in the catalytic properties of CuM@Fe3O4. In addition, the effects of various water matrix species and the results of reusability experiment, mineralization experiment, and ecotoxicity test exhibited that CuM@Fe3O4/PMS system possessed excellent practical application value.
− was the main reactive oxygen species (ROS) in CuM@Fe3O4/PMS system. A series of characterization experiments (pHpzc tests, XPS, H2-TPR, et al.) and DFT calculation disclosed that the addition of an unreducible metal M yielded many positive effects: (1) the formation of surface oxygen vacancies (OV) raised the zero point charge (pHpzc) of CuM@Fe3O4, thus enhanced the adsorption and activation of PMS; (2) promoting the generation of a new Cu species (Cu3+) on the surface of CuM@Fe3O4, which then participated in the generation of 1O2. The different reducibility of Cu3+ led to differences in the catalytic properties of CuM@Fe3O4. In addition, the effects of various water matrix species and the results of reusability experiment, mineralization experiment, and ecotoxicity test exhibited that CuM@Fe3O4/PMS system possessed excellent practical application value.
中文翻译:

通过结合不可还原的金属氧化物来调节铜/过硫酸盐的活化途径:表面氧空位的关键作用
在本文中,我们令人惊讶地发现不可还原的金属氧化物M x O y(M = Mg,Zn,Ca,Ba,Al)掺入CuO杂化磁性纳米三氧化二铁(Cu @ Fe 3 O 4)可能会改变反应过硫酸盐活化途径,并增加反应速率常数。Cu @ Fe 3 O 4对过氧一硫酸盐(PMS)的活化导致经典的基于硫酸根的氧化过程(SR-AOP),对乙酰氨基酚(ACE)降解速率常数为0.004 min -1,而1 O 2为主的非自由基CuM @ Fe 3 O 4中公开了氧化过程反应速率常数从0.003到0.242 min -1剧烈波动。机制研究表明,单重态氧(1 Ò 2从超氧化物阴离子自由基的直接氧化(O衍生)2 - )或的O-重组2 -是主要的活性氧物质(ROS)在CUM @的Fe 3 ö 4 / PMS系统。一系列表征实验(pH pzc测试,XPS,H 2 -TPR等)和DFT计算表明,添加不可还原金属M产生了许多积极作用:(1)表面氧空位(O V)的形成)提高零点电荷(pH
 PZC)的CUM @的Fe 3 ö 4,从而增强PMS的吸附和活化; (2)促进在CuM @ Fe 3 O 4表面生成新的Cu物种(Cu 3+),然后参与生成1 O 2。Cu 3+的不同还原性导致CuM @ Fe 3 O 4的催化性能不同。另外,各种水基质的影响以及可重复使用性试验,矿化试验和生态毒性试验的结果表明,CuM @ Fe 3 O 4/ PMS系统具有极好的实际应用价值。
PZC)的CUM @的Fe 3 ö 4,从而增强PMS的吸附和活化; (2)促进在CuM @ Fe 3 O 4表面生成新的Cu物种(Cu 3+),然后参与生成1 O 2。Cu 3+的不同还原性导致CuM @ Fe 3 O 4的催化性能不同。另外,各种水基质的影响以及可重复使用性试验,矿化试验和生态毒性试验的结果表明,CuM @ Fe 3 O 4/ PMS系统具有极好的实际应用价值。






































 京公网安备 11010802027423号
京公网安备 11010802027423号