当前位置:
X-MOL 学术
›
Inorg. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metal (Co/Mo)–N bond anchor-doped N in porous carbon for electrochemical nitrogen reduction
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2020-12-28 , DOI: 10.1039/d0qi01324b Yanyan Liu 1, 2, 3, 4, 5 , Shengbo Zhang 1, 2, 3, 4, 5 , Wenyi Li 1, 2, 3, 4, 5 , Hongjian Zhou 1, 2, 3, 4, 5 , Guozhong Wang 1, 2, 3, 4, 5 , Haimin Zhang 1, 2, 3, 4, 5
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2020-12-28 , DOI: 10.1039/d0qi01324b Yanyan Liu 1, 2, 3, 4, 5 , Shengbo Zhang 1, 2, 3, 4, 5 , Wenyi Li 1, 2, 3, 4, 5 , Hongjian Zhou 1, 2, 3, 4, 5 , Guozhong Wang 1, 2, 3, 4, 5 , Haimin Zhang 1, 2, 3, 4, 5
Affiliation
|
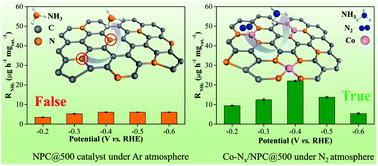
Currently, nitrogenous electrocatalysts (particularly, nitrogen (N)-doped carbon materials) have attracted extensive attention owing to their superior electrochemical nitrogen reduction reaction (NRR) performances. Unfortunately, doped nitrogen atoms could directly contribute to the formation of ammonia via dissociation and hydrogenation. Significantly, the type of N atoms (pyrrolic-N, pyridinic-N, or graphitic-N) in N-doped carbon donated to NH3 formation still needs to be verified. Here, using chitosan-derived N-doped porous carbon as an NRR electrocatalyst, we demonstrated that pyrrolic-N and pyridinic-N could disaggregate and hydrogenate to form NH3, leading to the increase in the yield of ammonia. To remedy this issue, metal–N bonds as an efficient and universal strategy were proposed to anchor doped N in porous carbon for NRR. Specifically, Co (Mo) atoms coordinated with N atoms could not only effectively inhibit the disaggregation of doped N atoms but also successfully promote the electrocatalytic NRR activity, thus yielding a boosted NRR performance.
中文翻译:

多孔碳中的金属(Co / Mo)–N键锚固掺杂氮,用于电化学还原氮
当前,含氮电催化剂(特别是掺氮(N)的碳材料)由于其优异的电化学氮还原反应(NRR)性能而引起了广泛关注。不幸的是,掺杂的氮原子可以通过离解和氢化直接促进氨的形成。值得注意的是,仍需验证捐赠给NH 3的N掺杂碳中N原子的类型(吡咯-N,吡啶-N或石墨-N)。在这里,使用壳聚糖衍生的氮掺杂多孔碳作为NRR电催化剂,我们证明了吡咯-N和吡啶-N可以分解并氢化形成NH 3,导致氨气产量增加。为了解决这个问题,有人提出了将金属-N键作为一种有效的通用策略,将掺杂的N固定在多孔碳中以实现NRR。具体地,与N原子配位的Co(Mo)原子不仅可以有效地抑制掺杂的N原子的解聚,而且可以成功地促进电催化NRR活性,从而产生增强的NRR性能。
更新日期:2021-01-20
中文翻译:

多孔碳中的金属(Co / Mo)–N键锚固掺杂氮,用于电化学还原氮
当前,含氮电催化剂(特别是掺氮(N)的碳材料)由于其优异的电化学氮还原反应(NRR)性能而引起了广泛关注。不幸的是,掺杂的氮原子可以通过离解和氢化直接促进氨的形成。值得注意的是,仍需验证捐赠给NH 3的N掺杂碳中N原子的类型(吡咯-N,吡啶-N或石墨-N)。在这里,使用壳聚糖衍生的氮掺杂多孔碳作为NRR电催化剂,我们证明了吡咯-N和吡啶-N可以分解并氢化形成NH 3,导致氨气产量增加。为了解决这个问题,有人提出了将金属-N键作为一种有效的通用策略,将掺杂的N固定在多孔碳中以实现NRR。具体地,与N原子配位的Co(Mo)原子不仅可以有效地抑制掺杂的N原子的解聚,而且可以成功地促进电催化NRR活性,从而产生增强的NRR性能。































 京公网安备 11010802027423号
京公网安备 11010802027423号