当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
1-Aminobicyclo[2.2.2]octane-2-carboxylic Acid and Derivatives As Chiral Constrained Bridged Scaffolds for Foldamers and Chiral Catalysts
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2021-01-19 , DOI: 10.1021/acs.accounts.0c00680 Pierre Milbeo 1 , Jean Martinez 1 , Muriel Amblard 1 , Monique Calmès 1 , Baptiste Legrand 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2021-01-19 , DOI: 10.1021/acs.accounts.0c00680 Pierre Milbeo 1 , Jean Martinez 1 , Muriel Amblard 1 , Monique Calmès 1 , Baptiste Legrand 1
Affiliation
|
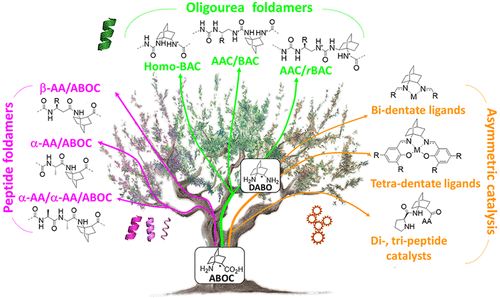
The improvement of molecular diversity is one of the major concerns of chemists since the continuous development of original synthetic molecules provides unique scaffolds usable in organic and bioorganic chemistry. The challenge is to develop versatile platforms with highly controlled chemical three-dimensional space thanks to controlled chirality and conformational restraints. In this respect, cyclic β-amino acids are of great interest with applications in various fields of chemistry. In addition to their intrinsic biological properties, they are important precursors for the synthesis of new generations of bioactive compounds such as antibiotics, enzyme inhibitors, and antitumor agents. They have also been involved in asymmetric synthesis as efficient organo-catalysts in their free form and as derivatives. Finally, constrained cyclic β-amino acids have been incorporated into oligomers to successfully stabilize original structures in foldamer science with recent successes in health, material science, and catalysis. Over the last ∼10 years, we focused on bicyclic β-amino acids possessing a bicyclo[2.2.2]octane structure. This latter is a structural key element in numerous families of biologically active natural and synthetic products and is an interesting template for asymmetric synthesis. Nonetheless, reported studies on bicyclic carbo-bridged compounds are rather limited compared to those on bicyclic-fused and heterobridged derivatives. In this Account, we particularly focused on the synthesis and applications of the 1-aminobicyclo[2.2.2]octane-2-carboxylic acid, named, ABOC, and its derivatives. This highly constrained bicyclic β-amino acid, with a sterically hindered bridgehead primary amine and an endocyclic chiral center, displays drastically reduced conformational freedom. In addition, its high bulkiness strongly impacts the spatial orientation of the appended functionalities and the conformation of adjacent building blocks. Thus, we have first expanded a fundamental synthetic work by a wide ranging study in the field of foldamers, in the design of various stable peptide/peptidomimetic helical structures incorporating the ABOC residue (11/9-, 18/16-, 12/14/14-, and 12/10-helices). In addition, such bicyclic residue was fully compatible with and stabilized the canonical oligourea helix, whereas very few cyclic β-amino acids have been incorporated into oligoureas. In addition, we have pursued with the synthesis of some ABOC derivatives, in particular the 1,2-diaminobicyclo[2.2.2]octane chiral diamine, named DABO, and its investigation in chiral catalytic systems. Covalent organo-catalysis of the aldol reaction using ABOC-containing tripeptide catalysts provided a range of aldol products with high enantioselectivity. Moreover, the double reductive condensation of DABO with various aldehydes allowed the building of new chiral ligands that proved their efficiency in the copper-catalyzed asymmetric Henry reaction.
中文翻译:

1-氨基双环[2.2.2]辛烷-2-羧酸及其衍生物作为折叠剂和手性催化剂的手性约束桥连骨架
分子多样性的改善是化学家的主要关注之一,因为原始合成分子的不断发展提供了可用于有机和生物有机化学的独特支架。面临的挑战是要通过受控的手性和构象约束来开发具有高度受控的化学三维空间的多功能平台。在这方面,环状β-氨基酸在化学的各个领域中的应用引起了极大的兴趣。除了其固有的生物学特性外,它们还是合成新一代生物活性化合物(如抗生素,酶抑制剂和抗肿瘤剂)的重要前体。它们还以游离形式和衍生物形式作为有效的有机催化剂参与了不对称合成。最后,受约束的环状β-氨基酸已被掺入低聚物中,以成功稳定折叠剂科学中的原始结构,并在健康,材料科学和催化领域取得了最新成功。在过去约10年中,我们重点研究了具有双环[2.2.2]辛烷结构的双环β-氨基酸。后者是许多具有生物活性的天然和合成产物家族的结构关键元素,并且是不对称合成的有趣模板。但是,与双环稠合和杂桥衍生物相比,双环碳桥化合物的报道研究相当有限。在此报告中,我们特别关注1-氨基双环[2.2.2]辛烷-2-羧酸(称为ABOC)及其衍生物的合成和应用。这种高度受限的双环β-氨基酸,具有位阻桥头伯胺和一个环内手性中心的化合物,其构象自由度大大降低。此外,它的高体积极大地影响了附加功能的空间方向和相邻构件的构象。因此,我们首先在折叠剂领域进行了广泛的研究,扩大了基础合成工作,设计了各种稳定的结合ABOC残基的肽/拟肽螺旋结构(11 / 9-,18 / 16-,12/14 / 14-和12/10螺旋)。另外,这种双环残基与典型的寡聚尿素螺旋完全相容并使其稳定,而很少有环状β-氨基酸被掺入到寡聚尿素中。另外,我们一直在追求一些ABOC衍生物的合成,特别是1,2-二氨基双环[2.2]。2]辛烷手性二胺,称为DABO,及其在手性催化体系中的研究。使用含ABOC的三肽催化剂对醛醇缩合反应进行共价有机催化,提供了一系列具有高对映选择性的醛醇缩合产物。此外,DABO与各种醛的双重还原缩合可以构建新的手性配体,证明了它们在铜催化的不对称亨利反应中的效率。
更新日期:2021-02-02
中文翻译:

1-氨基双环[2.2.2]辛烷-2-羧酸及其衍生物作为折叠剂和手性催化剂的手性约束桥连骨架
分子多样性的改善是化学家的主要关注之一,因为原始合成分子的不断发展提供了可用于有机和生物有机化学的独特支架。面临的挑战是要通过受控的手性和构象约束来开发具有高度受控的化学三维空间的多功能平台。在这方面,环状β-氨基酸在化学的各个领域中的应用引起了极大的兴趣。除了其固有的生物学特性外,它们还是合成新一代生物活性化合物(如抗生素,酶抑制剂和抗肿瘤剂)的重要前体。它们还以游离形式和衍生物形式作为有效的有机催化剂参与了不对称合成。最后,受约束的环状β-氨基酸已被掺入低聚物中,以成功稳定折叠剂科学中的原始结构,并在健康,材料科学和催化领域取得了最新成功。在过去约10年中,我们重点研究了具有双环[2.2.2]辛烷结构的双环β-氨基酸。后者是许多具有生物活性的天然和合成产物家族的结构关键元素,并且是不对称合成的有趣模板。但是,与双环稠合和杂桥衍生物相比,双环碳桥化合物的报道研究相当有限。在此报告中,我们特别关注1-氨基双环[2.2.2]辛烷-2-羧酸(称为ABOC)及其衍生物的合成和应用。这种高度受限的双环β-氨基酸,具有位阻桥头伯胺和一个环内手性中心的化合物,其构象自由度大大降低。此外,它的高体积极大地影响了附加功能的空间方向和相邻构件的构象。因此,我们首先在折叠剂领域进行了广泛的研究,扩大了基础合成工作,设计了各种稳定的结合ABOC残基的肽/拟肽螺旋结构(11 / 9-,18 / 16-,12/14 / 14-和12/10螺旋)。另外,这种双环残基与典型的寡聚尿素螺旋完全相容并使其稳定,而很少有环状β-氨基酸被掺入到寡聚尿素中。另外,我们一直在追求一些ABOC衍生物的合成,特别是1,2-二氨基双环[2.2]。2]辛烷手性二胺,称为DABO,及其在手性催化体系中的研究。使用含ABOC的三肽催化剂对醛醇缩合反应进行共价有机催化,提供了一系列具有高对映选择性的醛醇缩合产物。此外,DABO与各种醛的双重还原缩合可以构建新的手性配体,证明了它们在铜催化的不对称亨利反应中的效率。






























 京公网安备 11010802027423号
京公网安备 11010802027423号