Chemosphere ( IF 8.1 ) Pub Date : 2021-01-19 , DOI: 10.1016/j.chemosphere.2021.129683 Victoria Melin , Pablo Salgado , Abdoulaye Thiam , Adolfo Henríquez , Héctor D. Mansilla , Jorge Yáñez , Claudio Salazar
|
Amitriptyline (AMT) is the most widely used tricyclic antidepressant and is classified as a recalcitrant emergent contaminant because it has been detected in different sources of water. Its accumulation in water and soil represents a risk for different living creatures. To remove amitriptyline from wastewater, the Advanced Oxidation Processes (AOPs) stands up as an interesting option since generate highly oxidized species as hydroxyl radicals ( OH) by environmentally friendly mechanism. In this work, the oxidation and mineralization of AMT solution have been comparatively studied by 3 Electrochemical AOPs (EAOPs) where the
OH) by environmentally friendly mechanism. In this work, the oxidation and mineralization of AMT solution have been comparatively studied by 3 Electrochemical AOPs (EAOPs) where the  OH is produced by anodic oxidation of H2O (AO-H2O2), or by electro-Fenton (EF) or photoelectro-Fenton (PEF).
OH is produced by anodic oxidation of H2O (AO-H2O2), or by electro-Fenton (EF) or photoelectro-Fenton (PEF).
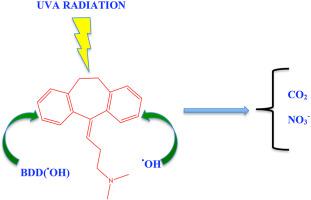
PEF process with a BDD anode showed the best performance for degradation and mineralization of this drug due to the synergistic action of highly reactive physiosorbed BDD ( OH), homogeneous
OH), homogeneous  OH and UVA radiation. This process achieved total degradation of AMT at 50 min of electrolysis and 95% of mineralization after 360 min of treatment with 0.5 mmol L−1 Fe2+ at 100 mA cm−2. Six aromatic intermediates for the drug mineralization were identified in short time of electrolysis by GC-MS, including a chloroaromatic by-product formed from the attack of active chlorine. Short-chain carboxylic acids like succinic, malic, oxalic and formic acid were quantified by ion-exclusion HPLC. Furthermore, the formation of NO3− ions was monitored. Finally, the organic intermediates identified by chromatographic techniques were used to propose the reaction sequence for the total mineralization of AMT.
OH and UVA radiation. This process achieved total degradation of AMT at 50 min of electrolysis and 95% of mineralization after 360 min of treatment with 0.5 mmol L−1 Fe2+ at 100 mA cm−2. Six aromatic intermediates for the drug mineralization were identified in short time of electrolysis by GC-MS, including a chloroaromatic by-product formed from the attack of active chlorine. Short-chain carboxylic acids like succinic, malic, oxalic and formic acid were quantified by ion-exclusion HPLC. Furthermore, the formation of NO3− ions was monitored. Finally, the organic intermediates identified by chromatographic techniques were used to propose the reaction sequence for the total mineralization of AMT.
中文翻译:

不同电化学高级氧化工艺降解阿米替林抗抑郁药的研究
阿米替林(AMT)是使用最广泛的三环抗抑郁药,由于已在不同的水源中检测到,因此被归类为顽固的紧急污染物。它在水和土壤中的积累代表着不同生物的风险。为了从废水中去除阿米替林,高级氧化工艺(AOPs)成为一个有趣的选择,因为它 通过环保机制生成高度氧化的物质,如羟基自由基(OH)。在这项工作中,已通过3种电化学AOP(EAOP)对AMT溶液的氧化和矿化进行了比较研究,其中
通过环保机制生成高度氧化的物质,如羟基自由基(OH)。在这项工作中,已通过3种电化学AOP(EAOP)对AMT溶液的氧化和矿化进行了比较研究,其中 OH是通过H 2 O(AO-H 2 O 2)的阳极氧化或通过Fenton电子(EF)生成的)或光电芬顿(PEF)。
OH是通过H 2 O(AO-H 2 O 2)的阳极氧化或通过Fenton电子(EF)生成的)或光电芬顿(PEF)。
具有BDD阳极的PEF工艺表现出该药物降解和矿化的最佳性能,这归因于高反应性物理吸附BDD( OH),均相
OH),均相 OH和UVA辐射的协同作用。在0.5 mA L -1 Fe 2+于100 mA cm -2处理360分钟后,该过程在电解50分钟时AMT完全降解,而矿化度达到95%。在短时间内通过GC-MS鉴定出六种用于药物矿化的芳香族中间体,包括由活性氯的侵蚀形成的氯代芳烃副产物。短链羧酸如琥珀酸,苹果酸,草酸和甲酸通过离子排阻HPLC定量。此外,NO的形成3 -监测离子。最后,通过色谱技术鉴定的有机中间体被用于提出AMT总矿化的反应顺序。
OH和UVA辐射的协同作用。在0.5 mA L -1 Fe 2+于100 mA cm -2处理360分钟后,该过程在电解50分钟时AMT完全降解,而矿化度达到95%。在短时间内通过GC-MS鉴定出六种用于药物矿化的芳香族中间体,包括由活性氯的侵蚀形成的氯代芳烃副产物。短链羧酸如琥珀酸,苹果酸,草酸和甲酸通过离子排阻HPLC定量。此外,NO的形成3 -监测离子。最后,通过色谱技术鉴定的有机中间体被用于提出AMT总矿化的反应顺序。































 京公网安备 11010802027423号
京公网安备 11010802027423号