当前位置:
X-MOL 学术
›
J. Chem. Educ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cu Plating with Sn and Subsequent Bronze Formation under Mild Conditions
Journal of Chemical Education ( IF 2.5 ) Pub Date : 2021-01-19 , DOI: 10.1021/acs.jchemed.0c00552 Takahiro Suzuki 1, 2 , Masayuki Inoue 3
Journal of Chemical Education ( IF 2.5 ) Pub Date : 2021-01-19 , DOI: 10.1021/acs.jchemed.0c00552 Takahiro Suzuki 1, 2 , Masayuki Inoue 3
Affiliation
|
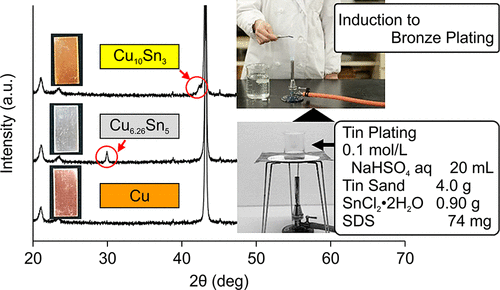
In a well-known bronze plating experiment, a Cu coupon is subjected to Sn plating and heating with a Bunsen burner. Currently, aqueous NaOH or HCl is typically used as the plating solution; however, the use of a strong acid or base provides operational risks, particularly in educational settings (e.g., schools). Herein, we introduce a modified method that uses aqueous NaHSO4 as the plating solution. This method reduces the operational risks and allows for the smooth deposition of silver-white Sn onto the Cu coupon in a higher yield than that obtained by the HCl method, which uses 30 times the concentration of the solute. When the Sn-plated Cu coupon is heated, it changes to bronze plating. This experiment is ideal for high school students, as it allows them to observe metal plating and solid diffusion.
中文翻译:

温和条件下镀锡和随后形成青铜的铜镀层
在众所周知的青铜镀覆实验中,对铜试样块进行镀锡,然后用本生灯进行加热。目前,通常使用NaOH或HCl水溶液作为电镀液。但是,使用强酸或强碱会带来操作风险,尤其是在教育环境(例如学校)中。在这里,我们介绍一种使用NaHSO 4水溶液作为电镀液的改进方法。该方法降低了操作风险,并允许将银白色Sn顺利沉积到Cu试样上,产率高于使用HCl方法的HCl方法(后者使用的是溶质浓度的30倍)。当镀锡的铜试样被加热时,它变成镀青铜。这个实验非常适合高中生,因为它可以让他们观察金属镀层和固体扩散。
更新日期:2021-03-09
中文翻译:

温和条件下镀锡和随后形成青铜的铜镀层
在众所周知的青铜镀覆实验中,对铜试样块进行镀锡,然后用本生灯进行加热。目前,通常使用NaOH或HCl水溶液作为电镀液。但是,使用强酸或强碱会带来操作风险,尤其是在教育环境(例如学校)中。在这里,我们介绍一种使用NaHSO 4水溶液作为电镀液的改进方法。该方法降低了操作风险,并允许将银白色Sn顺利沉积到Cu试样上,产率高于使用HCl方法的HCl方法(后者使用的是溶质浓度的30倍)。当镀锡的铜试样被加热时,它变成镀青铜。这个实验非常适合高中生,因为它可以让他们观察金属镀层和固体扩散。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号