当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Borrowing Carbonate-Enabled Allylic Amination Reactions under Additive- and Reductant-Free Nickel Catalysis Employing Allylic Alcohols
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2021-01-18 , DOI: 10.1021/acssuschemeng.0c08262 Gargi Nikhil Vaidya 1 , Mithilesh Nagpure 1 , Dinesh Kumar 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2021-01-18 , DOI: 10.1021/acssuschemeng.0c08262 Gargi Nikhil Vaidya 1 , Mithilesh Nagpure 1 , Dinesh Kumar 1
Affiliation
|
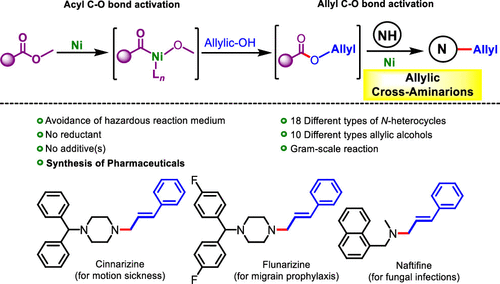
A “borrowing carbonate”-enabled allylic cross-amination reactions employing allylic alcohol were discovered via merging acyl/allyl C–O bonds activation under nickel catalysis. The key component of this protocol is the ability of nickel [Ni(II)-Ni(0)] to execute a relay process via the nucleophilic trapping of the generated acyl Ni complex, resulting from the acyl C–O bond cleavage of dialkyl carbonates, followed by selective allylic C–O bond activations (allylic C–O vs alkyl C–O vs acyl C–O) to yield π-allylNi-complexes. The finding truly represents Ni-catalyzed green allylic amination reactions under additive(s)-free conditions with excellent chemo- (N vs O), regio- (linear vs branched), and stereoselectivity (E vs Z) with a wide range of fundamentally challenging N-heterocycles and allylic alcohols. The reaction is scalable, does not require harmful reaction media and a globe box, and is successfully applied to the scale-up synthesis of pharmaceuticals (cinnarizine, flunarizine, and naftifine) with promising yields.
中文翻译:

在不含添加剂和不含还原剂的镍催化下利用烯丙醇借用碳酸酯使能的烯丙基胺化反应
通过在镍催化下合并酰基/烯丙基C-O键活化作用,发现了使用烯丙基醇进行“借入碳酸盐”的烯丙基交叉胺化反应。该协议的关键组成部分是镍[Ni(II)-Ni(0)]通过对碳酸二烷基酯的酰基C-O键裂解产生的酰基镍配合物进行亲核捕获来执行中继过程的能力然后进行选择性的烯丙基C–O键活化(烯丙基C–O与烷基C–O与酰基C–O)以生成π-烯丙基镍配合物。这一发现确实代表了Ni-催化的绿色烯丙基胺化反应在无添加剂的条件下具有优异的化学(N对O),区域(线性对支链)和立体选择性(E对Z),具有广泛的基本范围。挑战性N-杂环和烯丙醇。该反应具有可扩展性,不需要有害的反应介质和球形罩,并已成功应用于具有希望的收率的药物(肉桂那嗪,氟尿利嗪和萘替芬)的放大合成。
更新日期:2021-02-01
中文翻译:

在不含添加剂和不含还原剂的镍催化下利用烯丙醇借用碳酸酯使能的烯丙基胺化反应
通过在镍催化下合并酰基/烯丙基C-O键活化作用,发现了使用烯丙基醇进行“借入碳酸盐”的烯丙基交叉胺化反应。该协议的关键组成部分是镍[Ni(II)-Ni(0)]通过对碳酸二烷基酯的酰基C-O键裂解产生的酰基镍配合物进行亲核捕获来执行中继过程的能力然后进行选择性的烯丙基C–O键活化(烯丙基C–O与烷基C–O与酰基C–O)以生成π-烯丙基镍配合物。这一发现确实代表了Ni-催化的绿色烯丙基胺化反应在无添加剂的条件下具有优异的化学(N对O),区域(线性对支链)和立体选择性(E对Z),具有广泛的基本范围。挑战性N-杂环和烯丙醇。该反应具有可扩展性,不需要有害的反应介质和球形罩,并已成功应用于具有希望的收率的药物(肉桂那嗪,氟尿利嗪和萘替芬)的放大合成。































 京公网安备 11010802027423号
京公网安备 11010802027423号