当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interrupted Pyridine Hydrogenation: Asymmetric Synthesis of δ‐Lactams
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-01-18 , DOI: 10.1002/anie.202016771 Tobias Wagener 1 , Lukas Lückemeier 1 , Constantin G Daniliuc 1 , Frank Glorius 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-01-18 , DOI: 10.1002/anie.202016771 Tobias Wagener 1 , Lukas Lückemeier 1 , Constantin G Daniliuc 1 , Frank Glorius 1
Affiliation
|
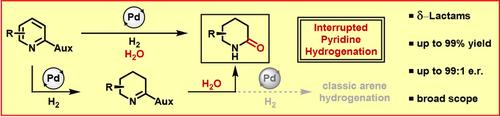
Metal‐catalyzed hydrogenation is an effective method to transform readily available arenes into saturated motifs, however, current hydrogenation strategies are limited to the formation of C−H and N−H bonds. The stepwise addition of hydrogen yields reactive unsaturated intermediates that are rapidly reduced. In contrast, the interruption of complete hydrogenation by further functionalization of unsaturated intermediates offers great potential for increasing chemical complexity in a single reaction step. Overcoming the tenet of full reduction in arene hydrogenation has been seldom demonstrated. In this work we report the synthesis of sought‐after, enantioenriched δ‐lactams from oxazolidinone‐substituted pyridines and water by an interrupted hydrogenation mechanism.
中文翻译:

吡啶间断氢化:δ-内酰胺的不对称合成
金属催化氢化是将容易获得的芳烃转化为饱和基序的有效方法,然而,目前的氢化策略仅限于形成C-H和N-H键。逐步添加氢气产生反应性不饱和中间体,并迅速还原。相比之下,通过不饱和中间体的进一步官能化来中断完全氢化为在单个反应步骤中增加化学复杂性提供了巨大的潜力。克服芳烃氢化完全还原的原则很少被证明。在这项工作中,我们报道了通过间断氢化机制从恶唑烷酮取代的吡啶和水合成广受欢迎的对映体富集的δ-内酰胺。
更新日期:2021-03-08
中文翻译:

吡啶间断氢化:δ-内酰胺的不对称合成
金属催化氢化是将容易获得的芳烃转化为饱和基序的有效方法,然而,目前的氢化策略仅限于形成C-H和N-H键。逐步添加氢气产生反应性不饱和中间体,并迅速还原。相比之下,通过不饱和中间体的进一步官能化来中断完全氢化为在单个反应步骤中增加化学复杂性提供了巨大的潜力。克服芳烃氢化完全还原的原则很少被证明。在这项工作中,我们报道了通过间断氢化机制从恶唑烷酮取代的吡啶和水合成广受欢迎的对映体富集的δ-内酰胺。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号