Molecular Metabolism ( IF 7.0 ) Pub Date : 2021-01-16 , DOI: 10.1016/j.molmet.2021.101168
Montserrat A de la Rosa Rodriguez 1 , Lei Deng 1 , Anne Gemmink 2 , Michel van Weeghel 3 , Marie Louise Aoun 4 , Christina Warnecke 5 , Rajat Singh 4 , Jan Willem Borst 6 , Sander Kersten 1
|
Objective
Storage of triglycerides in lipid droplets is governed by a set of lipid droplet-associated proteins. One of these lipid droplet-associated proteins, hypoxia-inducible lipid droplet-associated (HILPDA), was found to impair lipid droplet breakdown in macrophages and cancer cells by inhibiting adipose triglyceride lipase. Here, we aimed to better characterize the role and mechanism of action of HILPDA in hepatocytes.
Methods
We performed studies in HILPDA-deficient and HILPDA-overexpressing liver cells, liver slices, and mice. The functional role and physical interactions of HILPDA were investigated using a variety of biochemical and microscopic techniques, including real-time fluorescence live-cell imaging and Förster resonance energy transfer-fluorescence lifetime imaging microscopy (FRET-FLIM).
Results
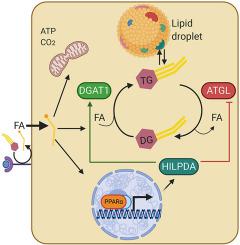
Levels of HILPDA were markedly induced by fatty acids in several hepatoma cell lines. Hepatocyte-specific deficiency of HILPDA in mice modestly but significantly reduced hepatic triglycerides in mice with non-alcoholic steatohepatitis. Similarly, deficiency of HILPDA in mouse liver slices and primary hepatocytes reduced lipid storage and accumulation of fluorescently-labeled fatty acids in lipid droplets, respectively, which was independent of adipose triglyceride lipase. Fluorescence microscopy showed that HILPDA partly colocalizes with lipid droplets and with the endoplasmic reticulum, is especially abundant in perinuclear areas, and mainly associates with newly added fatty acids. Real-time fluorescence live-cell imaging further revealed that HILPDA preferentially localizes to lipid droplets that are being remodeled. Overexpression of HILPDA in liver cells increased the activity of diacylglycerol acyltransferases (DGAT) and DGAT1 protein levels, concurrent with increased lipid storage. Confocal microscopy coupled to FRET-FLIM analysis demonstrated that HILPDA physically interacts with DGAT1 in living liver cells. The stimulatory effect of HILPDA on lipid storage via DGAT1 was corroborated in adipocytes.
Conclusions
Our data indicate that HILPDA physically interacts with DGAT1 and increases DGAT activity. Our findings suggest a novel regulatory mechanism by which fatty acids promote triglyceride synthesis and storage.
中文翻译:

低氧诱导的脂滴相关诱导 DGAT1 并促进肝细胞中的脂质储存
客观的
脂滴中甘油三酯的储存受一组脂滴相关蛋白的控制。其中一种脂滴相关蛋白,即低氧诱导型脂滴相关蛋白 (HILPDA),被发现通过抑制脂肪甘油三酯脂肪酶来损害巨噬细胞和癌细胞中的脂滴分解。在这里,我们旨在更好地描述 HILPDA 在肝细胞中的作用和作用机制。
方法
我们对 HILPDA 缺陷和 HILPDA 过度表达的肝细胞、肝切片和小鼠进行了研究。使用多种生化和显微技术研究了 HILPDA 的功能作用和物理相互作用,包括实时荧光活细胞成像和 Förster 共振能量转移-荧光寿命成像显微镜 (FRET-FLIM)。
结果
几种肝癌细胞系中的脂肪酸显着诱导了 HILPDA 水平。小鼠肝细胞特异性 HILPDA 缺陷适度但显着降低了非酒精性脂肪性肝炎小鼠的肝甘油三酯。同样,小鼠肝切片和原代肝细胞中 HILPDA 的缺乏分别减少了脂质储存和荧光标记脂肪酸在脂滴中的积累,这与脂肪甘油三酯脂肪酶无关。荧光显微镜显示HILPDA部分与脂滴和内质网共定位,在核周区域尤其丰富,主要与新添加的脂肪酸相关。实时荧光活细胞成像进一步显示 HILPDA 优先定位于正在重塑的脂滴。HILPDA 在肝细胞中的过表达增加了二酰基甘油酰基转移酶 (DGAT) 的活性和 DGAT1 蛋白水平,同时增加了脂质储存。共聚焦显微镜与 FRET-FLIM 分析相结合,表明 HILPDA 与活肝细胞中的 DGAT1 发生物理相互作用。HILPDA 通过 DGAT1 对脂质储存的刺激作用在脂肪细胞中得到证实。
结论
我们的数据表明 HILPDA 与 DGAT1 物理相互作用并增加 DGAT 活动。我们的研究结果表明了一种新的调节机制,脂肪酸通过该机制促进甘油三酯的合成和储存。

































 京公网安备 11010802027423号
京公网安备 11010802027423号