Earth-Science Reviews ( IF 10.8 ) Pub Date : 2021-01-17 , DOI: 10.1016/j.earscirev.2021.103525 Guang-Hui Yu , Yakov Kuzyakov
|
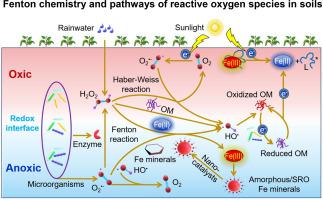
Although most organic matter (OM) in soil is mineralized by microorganisms, the nonmicrobial processes, e.g., Fenton reactions and photo-degradation, strongly contribute to OM decomposition and CO2 emission and are frequently the chemical background of biotic transformations. Fenton oxidation is a catalytic reaction chain of hydrogen peroxide (H2O2) with ferrous iron (Fe(II)) and Fe (oxyhydr)oxides that generates highly reactive hydroxyl radicals (HO•) oxidizing OM to CO2. Globally, reactive Fe (oxyhydr)oxides store at least one quarter (~600 Gt) of organic C in soil, which may be subjected to Fenton reactions, in which nano-sized Fe (oxyhydr)oxides act as nanocatalysts. The Fenton mechanisms depend on the sources and pathways of reactive oxygen species (ROS): O2•−, H2O2 and HO•. Given that microorganisms continuously produce ROS, biotic Fenton chemistry is ubiquitous in all soils (including subsoil), especially in those with strong UV radiation, fluctuating O2 concentrations and redox conditions, microbial hotspots such as rhizosphere and detritusphere, and high contents of amorphous or short-range ordered Fe (oxyhydr)oxides. Charcoal and biochar mediate heterogeneous catalysis and ROS formation in soil directly – as an electron shuttle – or indirectly by electron transfer from the valence band to the conduction band in the minerals under UV irradiation. Despite the extremely short lifetime (from nanoseconds to a few days), ROS are continuously produced and sustain the ubiquity of chelators and Fe(III) reduction. For the first time, we calculated the fundamental Eh-pH diagrams for ROS species and showed their relevance for Fenton reactions under specific soil conditions. Based on its extremely high reactivity (Eo = 2.8 V), HO• is one of the most powerful oxidants and may provide the most efficient energy release from Fenton reactions in soil. Even though the direct contribution of Fenton reactions to OM oxidation and CO2 emission is less than 0.5% on the global level, in some soils and ecosystems (e.g., hot deserts, red soils in the tropics and wet subtropics) it can reach 30% and even exceed 50% of total CO2 emissions. Fenton reactions are omnipresent and play a dual role for soil C cycling: they stimulate OM mineralization (including the most stable C pools such as charred C) and facilitate long-term C stabilization due to the increased recalcitrance of remaining OM and organo-mineral complex formation. Agricultural management positively affects Fenton reactions, accelerating C cycling and nutrient acquisition by plants. Accordingly, Fenton reactions and their effects on OM decomposition and formation are an emerging research field that explains the chemical background of many oxidative enzymatic processes. This may crucially change our views on C, energy and nutrient cycling in soils, especially in a changing world.
中文翻译:

Fenton化学和土壤中的活性氧:生物过程的非生物机制,控制以及碳和养分循环的后果
尽管土壤中的大多数有机物(OM)被微生物矿化,但非微生物过程(例如Fenton反应和光降解)强烈促进OM分解和CO 2排放,并且通常是生物转化的化学背景。Fenton氧化是过氧化氢(H 2 O 2)与亚铁离子(Fe(II))和Fe(羟基氧化物)的催化反应链,产生高反应性的羟基自由基(HO •),将OM氧化为CO 2。在全球范围内,反应性Fe(羟基氧化物)在土壤中存储至少四分之一(〜600 Gt)的有机碳,可进行Fenton反应,其中纳米级Fe(羟基氧化物)充当纳米催化剂。Fenton机制取决于活性氧(ROS)的来源和途径:O 2 •−,H 2 O 2和HO •。鉴于微生物不断产生ROS,Fenton生物化学在所有土壤(包括底土)中无处不在,特别是在紫外线辐射强烈,O 2波动的土壤中浓度和氧化还原条件,微生物热点(如根际和碎屑层)以及高含量的无定形或短程有序氧化铁(羟基氧化物)。木炭和生物炭直接介导土壤中的非均相催化和ROS的形成(作为电子穿梭),或在紫外线辐射下通过电子从矿物质的价带到导带的电子转移间接地介导。尽管寿命极短(从纳秒到几天),但仍会连续产生ROS,并保持螯合剂的普遍存在和Fe(III)的还原。我们首次计算了ROS物种的基本Eh-pH图,并显示了它们与特定土壤条件下Fenton反应的相关性。由于其极高的反应活性(E o = 2.8 V),HO •是最强大的氧化剂之一,可以从土壤中的Fenton反应中释放出最有效的能量。即使在全球范围内,芬顿反应对OM氧化和CO 2排放的直接贡献小于0.5%,但在某些土壤和生态系统中(例如,热沙漠,热带地区的红壤和亚热带湿润地区),Fenton反应仍可达到30%甚至超过总CO 2的50%排放。Fenton反应无处不在,并且在土壤碳循环中起着双重作用:它们会刺激OM矿化作用(包括最稳定的C池,例如烧焦的C),并且由于剩余OM和有机矿物质的顽固性增加而促进了长期C的稳定化。编队。农业管理对芬顿反应产生积极影响,加速了碳循环和植物吸收养分。因此,Fenton反应及其对OM分解和形成的影响是一个新兴的研究领域,它解释了许多氧化酶过程的化学背景。这可能会彻底改变我们对土壤中碳,能量和养分循环的看法,尤其是在不断变化的世界中。































 京公网安备 11010802027423号
京公网安备 11010802027423号