当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Seven-Step Stereodivergent Total Syntheses of Punicafolin and Macaranganin
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-01-14 , DOI: 10.1021/jacs.0c10714 Hiromitsu Shibayama 1 , Yoshihiro Ueda 1 , Takashi Tanaka 2 , Takeo Kawabata 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-01-14 , DOI: 10.1021/jacs.0c10714 Hiromitsu Shibayama 1 , Yoshihiro Ueda 1 , Takashi Tanaka 2 , Takeo Kawabata 1
Affiliation
|
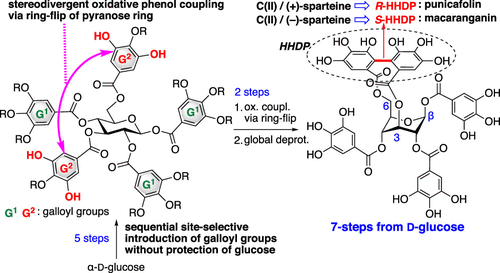
The first total syntheses of punicafolin (1) and macaranganin (2) were achieved in seven steps, respectively, from commercial α-d-glucose. The characteristic features of the synthesis are (1) sequential site-selective introduction of the adequate galloyl groups into unprotected d-glucose by a catalyst-controlled manner and (2) stereodivergent construction of the 3,6-HHDP bridge by oxidative phenol coupling of a common intermediate via a ring-flipping process of the glucose core. Because no protective groups were used for glucose throughout the process, extremely short-step total syntheses of natural glycosides 1 and 2 (MW 938) were performed.
中文翻译:

石榴苷和番石榴苷的七步立体发散全合成
第一次全合成石榴苷 (1) 和金刚鹦鹉 (2) 分别从商业 α-d-葡萄糖中分七个步骤完成。该合成的特征是(1)通过催化剂控制的方式将足够的没食子酰基顺序位点选择性地引入未保护的 d-葡萄糖中和(2)通过氧化苯酚偶联的 3,6-HHDP 桥的立体发散结构通过葡萄糖核心的环翻转过程的常见中间体。由于在整个过程中没有对葡萄糖使用保护基团,因此对天然糖苷 1 和 2 (MW 938) 进行了极短的全合成。
更新日期:2021-01-14
中文翻译:

石榴苷和番石榴苷的七步立体发散全合成
第一次全合成石榴苷 (1) 和金刚鹦鹉 (2) 分别从商业 α-d-葡萄糖中分七个步骤完成。该合成的特征是(1)通过催化剂控制的方式将足够的没食子酰基顺序位点选择性地引入未保护的 d-葡萄糖中和(2)通过氧化苯酚偶联的 3,6-HHDP 桥的立体发散结构通过葡萄糖核心的环翻转过程的常见中间体。由于在整个过程中没有对葡萄糖使用保护基团,因此对天然糖苷 1 和 2 (MW 938) 进行了极短的全合成。






























 京公网安备 11010802027423号
京公网安备 11010802027423号