当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding the Dechlorination of Chlorinated Hydrocarbons in the Pyrolysis of Mixed Plastics
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2021-01-15 , DOI: 10.1021/acssuschemeng.0c06461 Guozhan Jiang 1 , D. A. Sanchez Monsalve 2 , Peter Clough 3 , Ying Jiang 3 , Gary A. Leeke 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2021-01-15 , DOI: 10.1021/acssuschemeng.0c06461 Guozhan Jiang 1 , D. A. Sanchez Monsalve 2 , Peter Clough 3 , Ying Jiang 3 , Gary A. Leeke 1
Affiliation
|
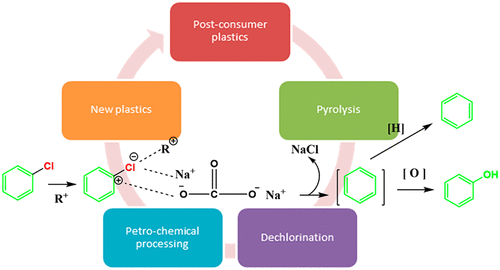
The dechlorination of chlorine containing hydrocarbons in pyrolysis vapor is poorly understood. In order to shed new light on the dechlorination mechanism, a model mixture composed of iso-octane doped with 2-chlorobutane, 2-chloroethylbenzene, and chlorobenzene was used to study the dechlorination of chlorinated hydrocarbons by alkali adsorption. These three chlorinated hydrocarbons were selected as they can be typically produced from the pyrolysis of mixed plastic waste containing polyvinyl chloride (PVC). The mixture is pumped continuously through a Na2CO3 or CaCO3/alumina bed, and GC-MS is used to identify the dechlorination products and to follow the dechlorination reactions. When chlorine is bonded to an aliphatic carbon with an adjacent aliphatic hydrogen, the chlorinated compound first undergoes a dehydrochlorination reaction to form HCl and olefins, and subsequently the HCl is reacted with the alkali in the absorbents. In our experiments, 2-chlorobutane is converted to 2-butene, and 2-chloroethylbenzene is converted to styrene. The formation of HCl and subsequent reaction with alkali components in the absorbent is verified by IR spectroscopy and XRD. In the presence of an alkali, the aliphatic chlorinated hydrocarbons underwent dechlorination at a temperature of 180 °C. The removal of chlorine from aromatic chlorinated compounds operates in a different mechanism, in which the C–Cl bond scission is promoted significantly by the presence of an alumina and hydrocarbon medium. It was found that chlorobenzene undergoes dechlorination forming phenol and benzene.
中文翻译:

了解混合塑料热解过程中氯化烃的脱氯作用
人们对热解蒸气中含氯碳氢化合物的脱氯知之甚少。为了阐明脱氯机理,使用了由异辛烷与2-氯丁烷,2-氯乙苯和氯苯组成的模型混合物来研究碱吸附对氯代烃的脱氯作用。选择这三种氯代烃是因为它们通常可以通过热分解含聚氯乙烯(PVC)的混合塑料废料来生产。将混合物连续泵送通过Na 2 CO 3或CaCO 3/氧化铝床,并使用GC-MS识别脱氯产物并跟踪脱氯反应。当氯与相邻的脂肪族氢键合到脂肪族碳上时,氯化化合物首先进行脱氯化氢反应以形成HCl和烯烃,然后HCl与吸收剂中的碱反应。在我们的实验中,2-氯丁烷被转化为2-丁烯,而2-氯乙苯被转化为苯乙烯。HCl的形成以及吸收剂中随后与碱成分的反应已通过红外光谱和XRD进行了验证。在碱的存在下,脂肪族氯化烃在180℃的温度下进行脱氯。从芳族氯化物中去除氯的机理不同,其中氧化铝和烃类介质的存在可显着促进C–Cl键的断裂。发现氯苯经历脱氯反应,形成苯酚和苯。
更新日期:2021-02-01
中文翻译:

了解混合塑料热解过程中氯化烃的脱氯作用
人们对热解蒸气中含氯碳氢化合物的脱氯知之甚少。为了阐明脱氯机理,使用了由异辛烷与2-氯丁烷,2-氯乙苯和氯苯组成的模型混合物来研究碱吸附对氯代烃的脱氯作用。选择这三种氯代烃是因为它们通常可以通过热分解含聚氯乙烯(PVC)的混合塑料废料来生产。将混合物连续泵送通过Na 2 CO 3或CaCO 3/氧化铝床,并使用GC-MS识别脱氯产物并跟踪脱氯反应。当氯与相邻的脂肪族氢键合到脂肪族碳上时,氯化化合物首先进行脱氯化氢反应以形成HCl和烯烃,然后HCl与吸收剂中的碱反应。在我们的实验中,2-氯丁烷被转化为2-丁烯,而2-氯乙苯被转化为苯乙烯。HCl的形成以及吸收剂中随后与碱成分的反应已通过红外光谱和XRD进行了验证。在碱的存在下,脂肪族氯化烃在180℃的温度下进行脱氯。从芳族氯化物中去除氯的机理不同,其中氧化铝和烃类介质的存在可显着促进C–Cl键的断裂。发现氯苯经历脱氯反应,形成苯酚和苯。































 京公网安备 11010802027423号
京公网安备 11010802027423号