当前位置:
X-MOL 学术
›
ACS Appl. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CoS2 Nanoparticles Supported on rGO, g-C3N4, BCN, MoS2, and WS2 Two-Dimensional Nanosheets with Excellent Electrocatalytic Performance for Overall Water Splitting: Electrochemical Studies and DFT Calculations
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2021-01-14 , DOI: 10.1021/acsaem.0c02509
Priyakshree Borthakur 1, 2 , Purna K. Boruah 1, 2 , Manash R. Das 1, 2 , Mohamed M. Ibrahim 3, 4 , Tariq Altalhi 3 , Hamdy S. El-Sheshtawy 4 , Sabine Szunerits 5 , Rabah Boukherroub 5 , Mohammed A. Amin 3, 6
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2021-01-14 , DOI: 10.1021/acsaem.0c02509
Priyakshree Borthakur 1, 2 , Purna K. Boruah 1, 2 , Manash R. Das 1, 2 , Mohamed M. Ibrahim 3, 4 , Tariq Altalhi 3 , Hamdy S. El-Sheshtawy 4 , Sabine Szunerits 5 , Rabah Boukherroub 5 , Mohammed A. Amin 3, 6
Affiliation
|
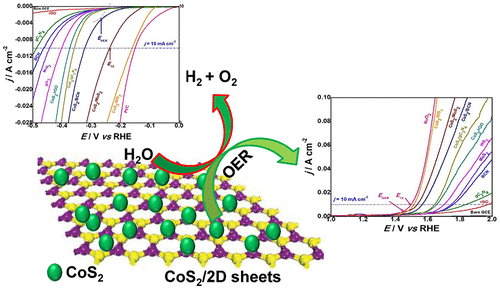
Efficient electrochemical splitting of water with exceptional durability can be a solution for growing global demand for energy. Herein, we systematically investigated the influence of the supporting two-dimensional (2D) substrate (rGO, g-C3N4, BCN, MoS2, and WS2) on the electrocatalytic performance of CoS2 nanoparticles (NPs) for overall water splitting. CoS2NPs decorated onto rGO, g-C3N4, BCN, MoS2, and WS2 sheets were synthesized by adopting a facile hydrothermal technique using cobalt salt and sulfur source as precursors. Compared to unsupported CoS2, the synthesized nanohybrids supported on 2D materials, namely, CoS2/rGO, CoS2/g-C3N4, CoS2/BCN, CoS2/MoS2, and CoS2/WS2 exhibited significantly higher water oxidation and reduction and overall water splitting efficiencies in 1.0 M KOH aqueous solution, with CoS2/MoS2 and CoS2/WS2 catalysts being the most effective ones. In the case of the hydrogen evolution reaction (HER), CoS2/WS2 performed as the best catalyst and was able to provide a current density of 10 mA cm–2 at an overpotential of 130 mV, whereas it only required 298 mV to generate the same current density for the oxygen evolution reaction (OER). The bifunctional nanohybrid CoS2/WS2 catalyst achieved a current density of 10 mA cm–2 over 96 h for the alkaline overall water splitting at a cell voltage of 1.6 V. Density functional theory (DFT) calculations were also performed to further describe and determine the HER catalytic performance of the studied catalysts. Comparing the absolute values of the studied catalysts’ Gibbs free energy of adsorbed hydrogen intermediate, H*, (|ΔGH*|), revealed that both CoS2/MoS2 and CoS2/WS2 hybrid catalysts exhibited the best surface stability and the highest HER catalytic performance.
中文翻译:

rGO,gC 3 N 4,BCN,MoS 2和WS 2二维纳米片支撑的CoS 2纳米颗粒具有优异的整体水分解电催化性能:电化学研究和DFT计算
具有卓越耐久性的高效电化学水分解可以成为不断增长的全球能源需求的解决方案。在本文中,我们系统地研究了支撑性二维(2D)基质(rGO,gC 3 N 4,BCN,MoS 2和WS 2)对CoS 2纳米颗粒(NPs)整体水分解的电催化性能的影响。采用钴盐和硫源为前驱体,采用简便的水热技术合成了装饰在rGO,gC 3 N 4,BCN,MoS 2和WS 2板上的CoS 2 NP 。与不受支持的CoS 2相比,负载在2D材料上的合成纳米杂化物,即CoS 2 / rGO,CoS 2 / gC 3 N 4,CoS 2 / BCN,CoS 2 / MoS 2和CoS 2 / WS 2表现出明显更高的水氧化和还原性,在1.0 M KOH水溶液中的水分解效率最高,其中CoS 2 / MoS 2和CoS 2 / WS 2催化剂最为有效。在析氢反应(HER)的情况下,CoS 2 / WS 2作为最佳催化剂,它可以在130 mV的超电势下提供10 mA cm –2的电流密度,而只需要298 mV即可产生与氧释放反应(OER)相同的电流密度。双功能纳米杂化CoS 2 / WS 2催化剂在电池电压为1.6 V的碱性总水分解过程中,在96小时内达到了10 mA cm –2的电流密度。还进行了密度泛函理论(DFT)计算以进一步描述和确定所研究催化剂的HER催化性能。比较所研究催化剂的吸附氢中间体H *(| ΔG H * |)的吉布斯自由能的绝对值,发现两种CoS2 / MoS 2和CoS 2 / WS 2杂化催化剂表现出最佳的表面稳定性和最高的HER催化性能。
更新日期:2021-02-22
中文翻译:

rGO,gC 3 N 4,BCN,MoS 2和WS 2二维纳米片支撑的CoS 2纳米颗粒具有优异的整体水分解电催化性能:电化学研究和DFT计算
具有卓越耐久性的高效电化学水分解可以成为不断增长的全球能源需求的解决方案。在本文中,我们系统地研究了支撑性二维(2D)基质(rGO,gC 3 N 4,BCN,MoS 2和WS 2)对CoS 2纳米颗粒(NPs)整体水分解的电催化性能的影响。采用钴盐和硫源为前驱体,采用简便的水热技术合成了装饰在rGO,gC 3 N 4,BCN,MoS 2和WS 2板上的CoS 2 NP 。与不受支持的CoS 2相比,负载在2D材料上的合成纳米杂化物,即CoS 2 / rGO,CoS 2 / gC 3 N 4,CoS 2 / BCN,CoS 2 / MoS 2和CoS 2 / WS 2表现出明显更高的水氧化和还原性,在1.0 M KOH水溶液中的水分解效率最高,其中CoS 2 / MoS 2和CoS 2 / WS 2催化剂最为有效。在析氢反应(HER)的情况下,CoS 2 / WS 2作为最佳催化剂,它可以在130 mV的超电势下提供10 mA cm –2的电流密度,而只需要298 mV即可产生与氧释放反应(OER)相同的电流密度。双功能纳米杂化CoS 2 / WS 2催化剂在电池电压为1.6 V的碱性总水分解过程中,在96小时内达到了10 mA cm –2的电流密度。还进行了密度泛函理论(DFT)计算以进一步描述和确定所研究催化剂的HER催化性能。比较所研究催化剂的吸附氢中间体H *(| ΔG H * |)的吉布斯自由能的绝对值,发现两种CoS2 / MoS 2和CoS 2 / WS 2杂化催化剂表现出最佳的表面稳定性和最高的HER催化性能。

































 京公网安备 11010802027423号
京公网安备 11010802027423号