Journal of Environmental Chemical Engineering ( IF 7.4 ) Pub Date : 2021-01-14 , DOI: 10.1016/j.jece.2021.105083 Bumgi Heo , Yong-Tae Kim , Jinsub Choi
|
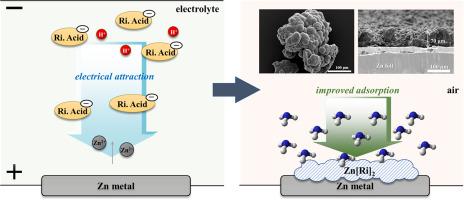
Zinc ricinoleate (Zn(Ri)2) exhibits excellent selectivity and the capability to remove odor-active compounds, such as nitrous compounds (NH3 and organic nitrogen) and sulfurous compounds (H2S, organic sulfides, and mercaptans). In contrast with the conventional catalyst-(or enzyme)-aided process, in this study, Zn(Ri)2 was prepared on zinc foil by a facile electrochemical approach. The electrochemically synthesized Zn(Ri)2 was characterized and compared to commercially available Zn(Ri)2, which clearly indicated that the average size and the production yield are strongly dependent on the external bias and anodization time: an increase in the diameter from 9.3 µm at 30 V to 15.2 µm at 80 V. In particular, an external bias of 80 V afforded the most stable particle structure and highest production yield. During anodization, the particles agglomerate and grow to form larger particles. With a distinct spherical morphology, the increased surface area contributes to the enhanced odor-removing capacity of 88% in 30 min, which is 2.3 times stronger than that of the commercial product.
中文翻译:

蓖麻油酸锌的电化学合成及其在氨吸附中的应用
蓖麻油酸锌(Zn(Ri)2)表现出出色的选择性,并具有去除异味活性化合物(如亚硝基化合物(NH 3和有机氮)和含硫化合物(H 2 S,有机硫化物和硫醇)的能力。与常规的催化剂(或酶)辅助工艺相反,在这项研究中,通过简便的电化学方法在锌箔上制备了Zn(Ri)2。表征了电化学合成的Zn(Ri)2并与市售Zn(Ri)2进行了比较,这清楚地表明平均尺寸和生产良率很大程度上取决于外部偏置和阳极氧化时间:直径从30 V的9.3 µm增加到80 V的15.2 µm。特别是80 V的外部偏置提供了最稳定的颗粒结构和最高的产量。在阳极氧化过程中,颗粒聚集成团并生长形成更大的颗粒。具有明显的球形形态,增加的表面积有助于在30分钟内提高88%的除臭能力,这比市售产品强2.3倍。








































 京公网安备 11010802027423号
京公网安备 11010802027423号