当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalyst-free generation of acyl radicals induced by visible light in water to construct C–N bonds
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2021-1-5 , DOI: 10.1039/d0ob02364g Maogang Ran 1, 2, 3, 4, 5 , Jiaxin He 1, 2, 3, 4, 5 , Boyu Yan 1, 2, 3, 4, 5 , Wenbo Liu 6, 7, 8, 9 , Yi Li 1, 2, 3, 4, 5 , Yunfen Fu 1, 2, 3, 4, 5 , Chao-Jun Li 6, 7, 8, 9 , Qiuli Yao 1, 2, 3, 4, 5
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2021-1-5 , DOI: 10.1039/d0ob02364g Maogang Ran 1, 2, 3, 4, 5 , Jiaxin He 1, 2, 3, 4, 5 , Boyu Yan 1, 2, 3, 4, 5 , Wenbo Liu 6, 7, 8, 9 , Yi Li 1, 2, 3, 4, 5 , Yunfen Fu 1, 2, 3, 4, 5 , Chao-Jun Li 6, 7, 8, 9 , Qiuli Yao 1, 2, 3, 4, 5
Affiliation
|
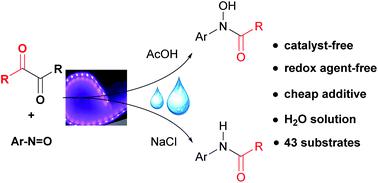
We describe herein a catalyst-free and redox-neutral photochemical strategy for the direct generation of acyl radicals from α-diketones, and its selective conversion of nitrosoarenes to hydroxyamides or amides with AcOH or NaCl as an additive. The reaction was carried out under mild conditions in water with purple LEDs as the light source. A broad scope of substrates was demonstrated. Mechanistic experiments indicate that α-diketones cleave to give acyl radicals, with hydroxyamides being further reduced to amides.
中文翻译:

水中可见光诱导形成C–N键的无催化剂酰基自由基的产生
我们在这里描述了无催化剂和氧化还原中性的光化学策略,用于直接从α-二酮生成酰基自由基,以及将亚硝基芳烃选择性转化为羟酰胺或酰胺,并以AcOH或NaCl作为添加剂的方法。该反应在温和的条件下在水中以紫色LED为光源进行。展示了广泛的基板范围。机理实验表明,α-二酮裂解产生酰基,而羟酰胺被进一步还原为酰胺。
更新日期:2021-01-13
中文翻译:

水中可见光诱导形成C–N键的无催化剂酰基自由基的产生
我们在这里描述了无催化剂和氧化还原中性的光化学策略,用于直接从α-二酮生成酰基自由基,以及将亚硝基芳烃选择性转化为羟酰胺或酰胺,并以AcOH或NaCl作为添加剂的方法。该反应在温和的条件下在水中以紫色LED为光源进行。展示了广泛的基板范围。机理实验表明,α-二酮裂解产生酰基,而羟酰胺被进一步还原为酰胺。































 京公网安备 11010802027423号
京公网安备 11010802027423号