当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rhodium-catalyzed carbonylative coupling of alkyl halides with thiols: a radical process faster than easier nucleophilic substitution
Chemical Communications ( IF 4.3 ) Pub Date : 2021-1-8 , DOI: 10.1039/d0cc07578g
Han-Jun Ai 1, 2, 3, 4 , Jabor Rabeah 1, 2, 3, 4 , Angelika Brückner 1, 2, 3, 4 , Xiao-Feng Wu 1, 2, 3, 4, 5
Chemical Communications ( IF 4.3 ) Pub Date : 2021-1-8 , DOI: 10.1039/d0cc07578g
Han-Jun Ai 1, 2, 3, 4 , Jabor Rabeah 1, 2, 3, 4 , Angelika Brückner 1, 2, 3, 4 , Xiao-Feng Wu 1, 2, 3, 4, 5
Affiliation
|
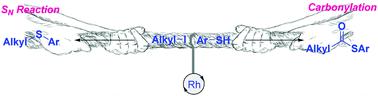
How to make a carbonylative coupling faster than the easier nucleophilic substitution? In this communication, a rhodium-catalyzed radical-based carbonylative coupling of alkyl halides with thiolphenols has been realized. Thioesters were isolated in good yields in general.
中文翻译:

铑催化卤代烷与硫醇的羰基偶联:比较容易的亲核取代要快的自由基过程
如何使羰基偶合比容易的亲核取代更快?在这种交流中,已经实现了烷基卤与硫醇酚的铑催化的基于自由基的羰基偶联。通常,以高收率分离出硫代酸酯。
更新日期:2021-01-13
中文翻译:

铑催化卤代烷与硫醇的羰基偶联:比较容易的亲核取代要快的自由基过程
如何使羰基偶合比容易的亲核取代更快?在这种交流中,已经实现了烷基卤与硫醇酚的铑催化的基于自由基的羰基偶联。通常,以高收率分离出硫代酸酯。

































 京公网安备 11010802027423号
京公网安备 11010802027423号