当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insight into Chemical Reduction and Charge Storage Mechanism of 2,2′-Dipyridyl Disulfide toward Stable Lithium–Organic Battery
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2021-01-13 , DOI: 10.1021/acs.jpclett.0c03496
Qianqian Fan 1 , Yubing Si 1 , Wei Guo 1 , Yongzhu Fu 1
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2021-01-13 , DOI: 10.1021/acs.jpclett.0c03496
Qianqian Fan 1 , Yubing Si 1 , Wei Guo 1 , Yongzhu Fu 1
Affiliation
|
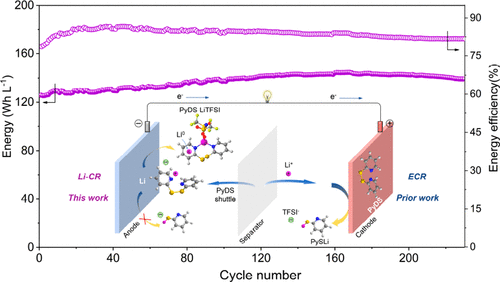
In lithium–organic batteries, organic cathode materials could dissolve in a liquid electrolyte and diffuse through the porous separator to the active lithium-metal anode, resulting in cycling instability. However, 2,2′-dipyridyl disulfide (PyDS) can be cycled 5 times better than diphenyl disulfide (PDS) although both are soluble. We believe this is related to their reactivity with lithium (Li0). Herein, we investigate the chemical reduction of PyDS by lithiated carbon paper (Li-CP) in ether electrolyte. It is found that only 6.3% of PyDS was reduced by Li-CP after 10 days, unlike PDS. Experimental and computational results show that PyDS molecules are ionized by lithium ions of lithium salts delocalizing the charge on pyridine rings of PyDS, which can momentarily store Li0, thus keeping the S–S bond inert in chemical reaction with Li0. This finding is successfully utilized in a membrane-free redox flow battery with PyDS catholyte, showing long cycle life with high energy density and energy efficiency. This work reveals the interesting charge storage mechanism and the different activity of organodisulfides toward electrochemical reduction and chemical reduction due to the organic groups, which can provide guidance for the design of stable lithium–organic batteries.
中文翻译:

2,2'-二吡啶二硫化物对稳定锂有机电池化学还原和电荷存储机理的研究
在锂有机电池中,有机阴极材料可能溶解在液体电解质中,并通过多孔隔板扩散到活性锂金属阳极,从而导致循环不稳定。但是,尽管2,2'-二吡啶基二硫化物(PyDS)都是可溶的,但其循环比二苯基二硫化物(PDS)好5倍。我们认为这与它们与锂(Li 0)的反应性有关。本文中,我们研究了锂化碳纸(Li-CP)在乙醚电解质中对PyDS的化学还原作用。发现与PDS不同,Li-CP在10天后仅还原了6.3%的PyDS。实验和计算结果表明,PyDS分子被锂盐中的锂离子电离,从而使PyDS吡啶环上的电荷离域,从而可以暂时存储Li 0因此,在与Li 0发生化学反应时,使S–S键保持惰性。这一发现已成功用于带有PyDS阴极液的无膜氧化还原液流电池,显示出长循环寿命以及高能量密度和能量效率。这项工作揭示了有趣的电荷存储机制以及有机二硫化物由于有机基团而对电化学还原和化学还原的不同活性,这可以为稳定的锂有机电池的设计提供指导。
更新日期:2021-01-21
中文翻译:

2,2'-二吡啶二硫化物对稳定锂有机电池化学还原和电荷存储机理的研究
在锂有机电池中,有机阴极材料可能溶解在液体电解质中,并通过多孔隔板扩散到活性锂金属阳极,从而导致循环不稳定。但是,尽管2,2'-二吡啶基二硫化物(PyDS)都是可溶的,但其循环比二苯基二硫化物(PDS)好5倍。我们认为这与它们与锂(Li 0)的反应性有关。本文中,我们研究了锂化碳纸(Li-CP)在乙醚电解质中对PyDS的化学还原作用。发现与PDS不同,Li-CP在10天后仅还原了6.3%的PyDS。实验和计算结果表明,PyDS分子被锂盐中的锂离子电离,从而使PyDS吡啶环上的电荷离域,从而可以暂时存储Li 0因此,在与Li 0发生化学反应时,使S–S键保持惰性。这一发现已成功用于带有PyDS阴极液的无膜氧化还原液流电池,显示出长循环寿命以及高能量密度和能量效率。这项工作揭示了有趣的电荷存储机制以及有机二硫化物由于有机基团而对电化学还原和化学还原的不同活性,这可以为稳定的锂有机电池的设计提供指导。

































 京公网安备 11010802027423号
京公网安备 11010802027423号