当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Deciphering the T790M/L858R-Selective Inhibition Mechanism of an Allosteric Inhibitor of EGFR: Insights from Molecular Simulations
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2021-01-12 , DOI: 10.1021/acschemneuro.0c00633
Miaomiao Li 1 , Jingjing Guo 1
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2021-01-12 , DOI: 10.1021/acschemneuro.0c00633
Miaomiao Li 1 , Jingjing Guo 1
Affiliation
|
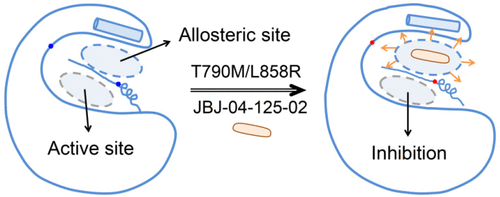
Allosteric inhibitors have lately received great attention because of their unique advantages, representing a more suitable choice for combinatory therapeutics targeting resistance-relevant signaling cascades. Among the various inhibitors, an allosteric small-molecule inhibitor, JBJ-04-125-02, has been proven to be effective against EGFRT790M/L858R mutant in vivo and in vitro. Herein, an in silico approach was adopted to shed light on the deep understanding of the higher selectivity of JBJ-04-125-02 against EGFRT790M/L858R mutant than wild-type EGFR. Our results indicate that JBJ-04-125-02 prefers to bind with the EGFRT790M/L858R mutant, stabilizes the inactive conformation, and further allosterically affects the conformations and dynamics of the interlobe cleft, including both the allosteric site and the ATP-binding site. Furthermore, docking results confirm that the binding of JBJ-04-125-02 at the allosteric site decreases the binding affinity of ANP (an ATP analogue) at the orthosteric site, especially for the Mut-holo one, which might further inhibit the function of EGFR. The present work provides a clear picture of the mutant-selective inhibition mechanism of an allosteric inhibitor of EGFR. The findings might pave the way for designing allosteric drugs targeting EGFR mutant lung cancer patients, which also takes a step forward in terms of drug resistance caused by protein mutations.
中文翻译:

破译EGFR别构抑制剂的T790M / L858R选择性抑制机制:分子模拟的见解。
变构抑制剂由于其独特的优势最近受到了广泛的关注,代表了针对靶向耐药相关信号传导级联的组合疗法的更合适的选择。在各种抑制剂中,变构小分子抑制剂JBJ-04-125-02已被证明在体内和体外均可有效对抗EGFR T790M / L858R突变体。在本文中,采用了计算机方法,以进一步了解JBJ-04-125-02对EGFR T790M / L858R突变体的选择性比野生型EGFR高。我们的结果表明JBJ-04-125-02倾向于与EGFR T790M / L858R结合突变体,稳定了非活性构象,并进一步变构影响了叶间裂的构象和动力学,包括变构位点和ATP结合位点。此外,对接结果证实,JBJ-04-125-02在变构位点的结合降低了正构位点的ANP(一种ATP类似物)的结合亲和力,尤其是对Mut-holo而言,这可能进一步抑制该功能EGFR。本工作提供了EGFR变构抑制剂的突变体选择性抑制机制的清晰画面。这些发现可能为设计针对EGFR突变型肺癌患者的变构药物铺平了道路,这在蛋白质突变引起的耐药性方面也向前迈进了一步。
更新日期:2021-02-03
中文翻译:

破译EGFR别构抑制剂的T790M / L858R选择性抑制机制:分子模拟的见解。
变构抑制剂由于其独特的优势最近受到了广泛的关注,代表了针对靶向耐药相关信号传导级联的组合疗法的更合适的选择。在各种抑制剂中,变构小分子抑制剂JBJ-04-125-02已被证明在体内和体外均可有效对抗EGFR T790M / L858R突变体。在本文中,采用了计算机方法,以进一步了解JBJ-04-125-02对EGFR T790M / L858R突变体的选择性比野生型EGFR高。我们的结果表明JBJ-04-125-02倾向于与EGFR T790M / L858R结合突变体,稳定了非活性构象,并进一步变构影响了叶间裂的构象和动力学,包括变构位点和ATP结合位点。此外,对接结果证实,JBJ-04-125-02在变构位点的结合降低了正构位点的ANP(一种ATP类似物)的结合亲和力,尤其是对Mut-holo而言,这可能进一步抑制该功能EGFR。本工作提供了EGFR变构抑制剂的突变体选择性抑制机制的清晰画面。这些发现可能为设计针对EGFR突变型肺癌患者的变构药物铺平了道路,这在蛋白质突变引起的耐药性方面也向前迈进了一步。

































 京公网安备 11010802027423号
京公网安备 11010802027423号