当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multitarget Biological Profiling of New Naphthoquinone and Anthraquinone-Based Derivatives for the Treatment of Alzheimer’s Disease
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2021-01-11 , DOI: 10.1021/acschemneuro.0c00624 Marta Campora 1 , Claudio Canale 2 , Elena Gatta 2 , Bruno Tasso 1 , Erik Laurini 3 , Annalisa Relini 2 , Sabrina Pricl 3, 4 , Marco Catto 5 , Michele Tonelli 1
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2021-01-11 , DOI: 10.1021/acschemneuro.0c00624 Marta Campora 1 , Claudio Canale 2 , Elena Gatta 2 , Bruno Tasso 1 , Erik Laurini 3 , Annalisa Relini 2 , Sabrina Pricl 3, 4 , Marco Catto 5 , Michele Tonelli 1
Affiliation
|
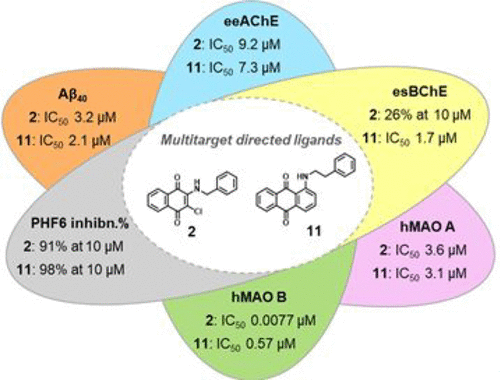
Two series of naphthoquinone and anthraquinone derivatives decorated with an aromatic/heteroaromatic chain have been synthesized and evaluated as potential promiscuous agents capable of targeting different factors playing a key role in Alzheimer’s disease (AD) pathogenesis. On the basis of the in vitro biological profiling, most of them exhibited a significant ability to inhibit amyloid aggregation, PHF6 tau sequence aggregation, acetylcholinesterase (AChE), and monoamine oxidase (MAO) B. In particular, naphthoquinone 2 resulted as one of the best performing multitarget-directed ligand (MTDL) experiencing a high potency profile in inhibiting β-amyloid (Aβ40) aggregation (IC50 = 3.2 μM), PHF6 tau fragment (91% at 10 μM), AChE enzyme (IC50 = 9.2 μM) jointly with a remarkable inhibitory activity against MAO B (IC50 = 7.7 nM). Molecular modeling studies explained the structure–activity relationship (SAR) around the binding modes of representative compound 2 in complex with hMAO B and hAChE enzymes, revealing inhibitor/protein key contacts and the likely molecular rationale for enzyme selectivity. Compound 2 was also demonstrated to be a strong inhibitor of Aβ42 aggregation, with potency comparable to quercetin. Accordingly, atomic force microscopy (AFM) revealed that the most promising naphthoquinones 2 and 5 and anthraquinones 11 and 12 were able to impair Aβ42 fibrillation, deconstructing the morphologies of its fibrillar aggregates. Moreover, the same compounds exerted a moderate neuroprotective effect against Aβ42 toxicity in primary cultures of cerebellar granule cells. Therefore, our findings demonstrate that these molecules may represent valuable chemotypes toward the development of promising candidates for AD therapy.
中文翻译:

新的基于萘醌和蒽醌的衍生物的多靶标生物学分析用于治疗阿尔茨海默氏病
已经合成并装饰了芳香族/杂芳香族链修饰的两个系列的萘醌和蒽醌衍生物,它们是潜在的混杂剂,能够靶向在阿尔茨海默氏病(AD)发病机理中起关键作用的不同因子。根据体外生物学分析,它们大多数显示出显着的抑制淀粉样蛋白聚集,PHF6 tau序列聚集,乙酰胆碱酯酶(AChE)和单胺氧化酶(MAO)B的能力。特别是,萘醌2是其中的一种表现最佳的多目标定向配体(MTDL)经历在抑制β淀粉样蛋白具有高效力轮廓(Aβ 40)聚集(IC 50= 3.2μM),PHF6 tau片段(在10μM时为91%),AChE酶(IC 50 = 9.2μM )对MAO B具有显着的抑制活性(IC 50 = 7.7 nM)。分子建模研究解释了与hMAO B和hAChE酶复合的代表性化合物2结合模式周围的结构-活性关系(SAR),揭示了抑制剂/蛋白质关键接触以及酶选择性的可能分子原理。还证明了化合物2是Aβ42聚集的强抑制剂,效力与槲皮素相当。因此,原子力显微镜(AFM)揭示了最有前途的萘醌2和5以及蒽醌11和12能够削弱Aβ42的原纤维形成,从而解构其原纤维聚集体的形态。此外,相同的化合物在小脑颗粒细胞的原代培养物中对Aβ42毒性具有中等程度的神经保护作用。因此,我们的发现表明,这些分子可能代表着有价值的化学型,可用于发展有希望的AD治疗候选药物。
更新日期:2021-02-03
中文翻译:

新的基于萘醌和蒽醌的衍生物的多靶标生物学分析用于治疗阿尔茨海默氏病
已经合成并装饰了芳香族/杂芳香族链修饰的两个系列的萘醌和蒽醌衍生物,它们是潜在的混杂剂,能够靶向在阿尔茨海默氏病(AD)发病机理中起关键作用的不同因子。根据体外生物学分析,它们大多数显示出显着的抑制淀粉样蛋白聚集,PHF6 tau序列聚集,乙酰胆碱酯酶(AChE)和单胺氧化酶(MAO)B的能力。特别是,萘醌2是其中的一种表现最佳的多目标定向配体(MTDL)经历在抑制β淀粉样蛋白具有高效力轮廓(Aβ 40)聚集(IC 50= 3.2μM),PHF6 tau片段(在10μM时为91%),AChE酶(IC 50 = 9.2μM )对MAO B具有显着的抑制活性(IC 50 = 7.7 nM)。分子建模研究解释了与hMAO B和hAChE酶复合的代表性化合物2结合模式周围的结构-活性关系(SAR),揭示了抑制剂/蛋白质关键接触以及酶选择性的可能分子原理。还证明了化合物2是Aβ42聚集的强抑制剂,效力与槲皮素相当。因此,原子力显微镜(AFM)揭示了最有前途的萘醌2和5以及蒽醌11和12能够削弱Aβ42的原纤维形成,从而解构其原纤维聚集体的形态。此外,相同的化合物在小脑颗粒细胞的原代培养物中对Aβ42毒性具有中等程度的神经保护作用。因此,我们的发现表明,这些分子可能代表着有价值的化学型,可用于发展有希望的AD治疗候选药物。

































 京公网安备 11010802027423号
京公网安备 11010802027423号