当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quantitative Insights into the Reaction Mechanism for the Direct Synthesis of H2O2 over Transition Metals: Coverage-Dependent Microkinetic Modeling
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-01-11 , DOI: 10.1021/acscatal.0c04125
Zihao Yao 1 , Jinyan Zhao 1 , Rhys J. Bunting 2 , Chenxia Zhao 1 , Peijun Hu 2 , Jianguo Wang 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-01-11 , DOI: 10.1021/acscatal.0c04125
Zihao Yao 1 , Jinyan Zhao 1 , Rhys J. Bunting 2 , Chenxia Zhao 1 , Peijun Hu 2 , Jianguo Wang 1
Affiliation
|
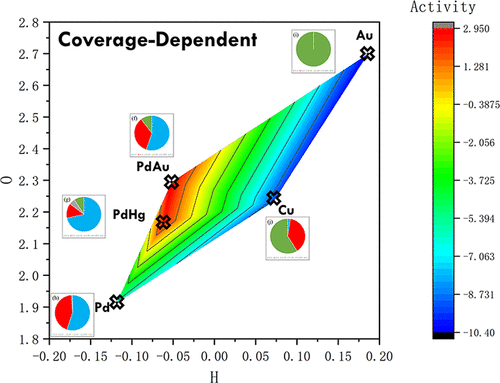
The direct synthesis is the most promising alternative method for the production of hydrogen peroxide, and the bottleneck is still unsolved. The breakthrough lies in elusive reaction mechanism issues. In this work, advanced coverage-dependent kinetic modeling is combined with the energetics from first-principles calculations to investigate the formation of H2O2 over transition metals. We show that the adsorbate–adsorbate interactions considerably affect the reaction mechanism of synthesis of hydrogen peroxide on Pd(111). Without the coverage effect, O2 is likely to go through the direct dissociation mechanism, and water is the major product. When the coverage effects are included, the dissociations of O–O and O–OH bonds are significantly inhibited, and on the contrary, the hydrogenations of O2 and OOH are promoted, leading to the production of H2O2. We demonstrate that the reaction temperature induces strong variations in the coverage of intermediates, which in turn causes changes in product selectivity. Being consistent with the operando experiment, our kinetic simulations indicate that the H2/O2 partial pressure ratio has great effects on H2O2 selectivity and the reaction rate of H2O2 is lower under hydrogen-rich (oxygen-lean) and oxygen-rich (hydrogen-lean) conditions, which is highly related to the intermediate coverage. The same approach is also applied to other important relevant metals, i.e., Cu(111), Au(111), PdAu, and PdHg alloys, and the trends of activity and selectivity have been obtained.
中文翻译:

过渡金属直接合成H 2 O 2的反应机理的定量见解:覆盖率依赖的微动力学模型
直接合成是生产过氧化氢的最有希望的替代方法,并且瓶颈仍未解决。突破在于难以捉摸的反应机制问题。在这项工作中,先进的依赖于覆盖的动力学模型与第一性原理计算的能量学相结合,以研究过渡金属上H 2 O 2的形成。我们表明,吸附物-吸附物的相互作用极大地影响了过氧化氢在Pd(111)上合成的反应机理。没有覆盖效应,O 2可能会经历直接解离机制,水是主要产物。当包括覆盖效应时,O-O和O-OH键的离解被显着抑制,相反,促进了O 2和OOH的氢化,从而导致H 2 O 2的产生。我们证明了反应温度引起中间体覆盖率的强烈变化,从而引起产物选择性的变化。与操作实验一致,我们的动力学模拟表明H 2 / O 2分压比对H 2 O 2的选择性和H 2的反应速率有很大的影响。O 2在富氢(贫氧)和富氧(贫氢)条件下较低,这与中间覆盖率高度相关。同样的方法也适用于其他重要的相关金属,例如Cu(111),Au(111),PdAu和PdHg合金,并且已经获得了活性和选择性的趋势。
更新日期:2021-02-05
中文翻译:

过渡金属直接合成H 2 O 2的反应机理的定量见解:覆盖率依赖的微动力学模型
直接合成是生产过氧化氢的最有希望的替代方法,并且瓶颈仍未解决。突破在于难以捉摸的反应机制问题。在这项工作中,先进的依赖于覆盖的动力学模型与第一性原理计算的能量学相结合,以研究过渡金属上H 2 O 2的形成。我们表明,吸附物-吸附物的相互作用极大地影响了过氧化氢在Pd(111)上合成的反应机理。没有覆盖效应,O 2可能会经历直接解离机制,水是主要产物。当包括覆盖效应时,O-O和O-OH键的离解被显着抑制,相反,促进了O 2和OOH的氢化,从而导致H 2 O 2的产生。我们证明了反应温度引起中间体覆盖率的强烈变化,从而引起产物选择性的变化。与操作实验一致,我们的动力学模拟表明H 2 / O 2分压比对H 2 O 2的选择性和H 2的反应速率有很大的影响。O 2在富氢(贫氧)和富氧(贫氢)条件下较低,这与中间覆盖率高度相关。同样的方法也适用于其他重要的相关金属,例如Cu(111),Au(111),PdAu和PdHg合金,并且已经获得了活性和选择性的趋势。

































 京公网安备 11010802027423号
京公网安备 11010802027423号