Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Complementary and Regioselective Synthesis of Isomeric 3-[Isoxazol-3(or 5)-yl]indoles from β-Ethylthio-β-indolyl-α,β-unsaturated Ketones
Synthesis ( IF 2.2 ) Pub Date : 2021-01-11 , DOI: 10.1055/s-0040-1706658 Haifeng Yu 1 , Wenju Wang 1 , Kehua Wang 2 , Xue Zhang 1
中文翻译:

由β-乙硫基-β-吲哚基-α,β-不饱和酮互补和区域选择性合成异构体3- [异恶唑-3(或5)-基]吲哚
更新日期:2021-01-12
Synthesis ( IF 2.2 ) Pub Date : 2021-01-11 , DOI: 10.1055/s-0040-1706658 Haifeng Yu 1 , Wenju Wang 1 , Kehua Wang 2 , Xue Zhang 1
Affiliation
|
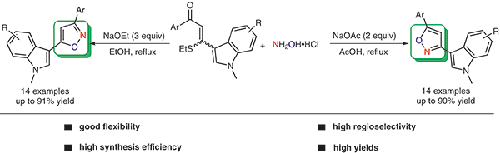
A simple and efficient method for the complementary and regioselective synthesis of isomeric 3-(isoxazol-5-yl)indoles and 3-(isoxazol-3-yl)indoles has been developed by the regioselective cyclocondensation reaction of β-ethylthio-β-indolyl-α,β-unsaturated ketones and hydroxylamine hydrochloride. It was found that the cyclocondensation reaction in the presence of excess NaOEt in refluxing EtOH gives 3-(isoxazol-5-yl)indoles in good yields, whereas using NaOAc in boiling AcOH gives 3-(isoxazol-3-yl)indoles in good yields.
中文翻译:

由β-乙硫基-β-吲哚基-α,β-不饱和酮互补和区域选择性合成异构体3- [异恶唑-3(或5)-基]吲哚
通过β-乙硫基-β-吲哚基的区域选择性环缩合反应,开发了一种简单有效的方法,用于互补和区域选择性合成异构体3-(异恶唑-5-基)吲哚和3-(异恶唑-3-基)吲哚。 -α,β-不饱和酮和盐酸羟胺。已经发现,在过量的NaOEt存在下,在回流的EtOH中进行环缩合反应,可以得到高产率的3-(异恶唑-5-基)吲哚,而在沸腾的AcOH中使用NaOAc可以得到良好的3-(异恶唑-3-基)吲哚。产量。








































 京公网安备 11010802027423号
京公网安备 11010802027423号