Molecular Catalysis ( IF 3.9 ) Pub Date : 2021-01-12 , DOI: 10.1016/j.mcat.2020.111356 Ahmed Ismail , Mingyang Li , Muhammad Zahid , Liman Fan , Cheng Zhang , Zhibin Li , Yujun Zhu
|
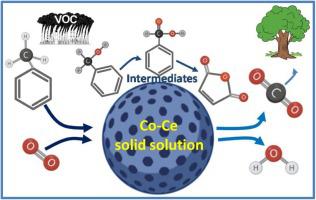
A series of CoxCe1-xO2-δ (x = 0.05−0.6) oxides were synthesized through non-ionic surfactant hydrothermal method and applied for catalytic toluene oxidation. Various characterizations were performed to investigate the relation between structure and activity of the catalysts, including XRD, N2 adsorption-desorption, Raman spectra, TEM, H2-TPR, O2-TPD, XPS and in situ DRIFTS. CoxCe1-xO2-δ shows higher catalytic performance than pure Co3O4 and CeO2, meanwhile Co0.2Ce0.8O2-δ exhibits the best catalytic activity for toluene oxidation in the presence and absence of water, moreover, no obvious difference in activity among three successive testing runs. This good activity can be mainly attributed to the strong interaction between Co and Ce oxides to form Co0.2Ce0.8O2-δ solid solution, leading to abundant surface active oxygen species, more surface Co3+ and Ce3+ species. In situ DRIFTS is used to probe the catalytic reaction process, which reveals that toluene can be rapidly adsorbed and transferred to benzoate species, finally oxidized into CO2 and H2O.
中文翻译:

Co x Ce 1-x O2 -δ氧化物中Co和Ce氧化物之间的强相互作用对其甲苯催化氧化的影响
通过非离子表面活性剂水热法合成了一系列Co x Ce 1-x O2 -δ(x = 0.05-0.6)氧化物,并用于催化甲苯氧化。进行了各种表征以研究催化剂的结构与活性之间的关系,包括XRD,N 2吸附-解吸,拉曼光谱,TEM,H 2 -TPR,O 2 -TPD,XPS和原位DRIFTS。Co x Ce 1-x O2 -δ表现出比纯Co 3 O 4和CeO 2更高的催化性能,而Co 0.2 Ce 0.8 O在有水和无水的情况下,2-δ表现出对甲苯氧化的最佳催化活性,而且在三个连续的测试运行中,活性没有明显差异。这种良好的活性主要归因于Co和Ce氧化物之间的强相互作用,形成了Co 0.2 Ce 0.8 O2 -δ固溶体,从而导致了丰富的表面活性氧种类,更多的表面Co 3+和Ce 3+种类。原位DRIFTS被用于探测催化反应过程中,这表明,甲苯可以迅速地吸附并转移至苯甲酸物种,最后氧化成CO 2和H 2 O.

































 京公网安备 11010802027423号
京公网安备 11010802027423号