European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2021-01-12 , DOI: 10.1016/j.ejmech.2021.113161 Mengwu Xiao , Lvjie Xu , Ding Lin , Wenwen Lian , Manying Cui , Meng Zhang , Xiaowei Yan , Shuishi Li , Jun Zhao , Jiao Ye , Ailin Liu , Aixi Hu
|
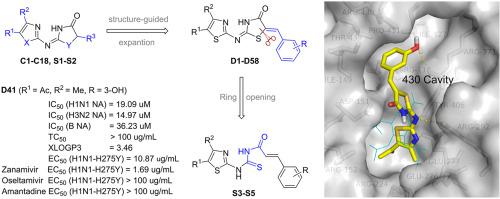
A series of 4-thiazolinone derivatives (D1-D58) were designed and synthesized. All of the derivatives were evaluated in vitro for neuraminidase (NA) inhibitory activities against influenza virus A (H1N1), and the inhibitory activities of the five most potent compounds were further evaluated on NA from two different influenza viral subtypes (H3N2 and B), and then their in vitro anti-viral activities were evaluated using the cytopathic effect (CPE) reduction assay. The results showed that the majority of the target compounds exhibited moderate to good NA inhibitory activity. Compound D18 presented the most potent inhibitory activity with IC50 values of 13.06 μM against influenza H1N1 subtype. Among the selected compounds, D18 and D41 turned out to be the most potent inhibitors against influenza virus H3N2 subtype (IC50 = 15.00 μM and IC50 = 14.97 μM, respectively). D25 was the most potent compound against influenza B subtype (IC50 = 16.09 μM). In addition, D41 showed low toxicity and greater potency than reference compounds Oseltamivir and Amantadine against N1-H275Y variant in cellular assays. The structure-activity relationship (SAR) analysis showed that introducing 4-CO2H, 4-OH, 3-OCH3-4-OH substituted benzyl methylene can greatly improve the activity of 4-thiazolinones. Further SAR analysis indicated that 4-thiazolinone and ferulic acid fragments are necessary fragments of target compounds for inhibiting NA. Molecular docking was performed to study the interaction between compound D41 and the active site of NA. This study may providing important information for new drug development for anti-influenza virus including mutant influenza virus.
中文翻译:

设计,合成和生物测定作为流感神经氨酸酶抑制剂的4-噻唑啉酮衍生物
设计并合成了一系列4-噻唑啉酮衍生物(D1-D58)。在体外评估了所有衍生物对甲型流感病毒(H1N1)的神经氨酸酶(NA)抑制活性,并进一步评估了来自两种不同流感病毒亚型(H3N2和B)的五种最有效化合物对NA的抑制活性,然后使用细胞病变效应(CPE)降低法评估其体外抗病毒活性。结果表明,大多数目标化合物均显示出中等至良好的NA抑制活性。化合物D18对IC 50的抑制作用最强H1N1流感亚型的抗药性值为13.06μM。中所选择的化合物,D18和D41被证明是针对流感病毒H3N2亚型的最有效的抑制剂(IC 50 = 15.00μM和IC 50 = 14.97μM,分别地)。D25是对抗B型流感最有效的化合物(IC 50 = 16.09μM)。此外,在细胞测定中,D41对N1-H275Y变体显示出比参考化合物Oseltamivir和Amantadine低的毒性和更高的效力。结构-活性关系(SAR)分析表明,引入4-CO 2 H,4-OH,3-OCH 3-4-OH取代的苄基亚甲基可以大大提高4-噻唑啉酮的活性。进一步的SAR分析表明4-噻唑啉酮和阿魏酸片段是抑制NA的目标化合物的必要片段。进行分子对接以研究化合物D41和NA的活性位点之间的相互作用。该研究可为包括突变型流感病毒在内的抗流感病毒新药开发提供重要信息。

































 京公网安备 11010802027423号
京公网安备 11010802027423号