Carbon ( IF 10.5 ) Pub Date : 2021-01-11 , DOI: 10.1016/j.carbon.2021.01.008 Pengfei Liu , Yanhui Yang , Qiang Wang
|
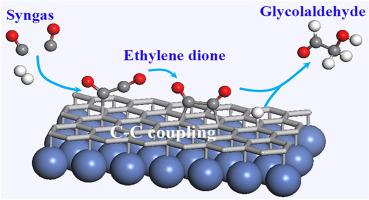
A critical step toward the rational design of new catalyst that achieve selective and efficient synthesis of C2+ oxygenates from syngas (CO/H2) by Fischer-Tropsch synthesis (FTS) is to determine the detailed reaction mechanism. Herein, the mechanism of two gaseous CO monomers coupling into a chemisorbed ethylene dione (O∗C∗CO) and subsequent hydrogenation of O∗C∗CO on the chainmail catalyst of nickel-supported graphene surface is reported. The results show that two gaseous CO monomers can be coupled into a two-atom-chemisorbed O∗C∗CO via a metastable intermediate of O∗CCO single-atom-chemisorbed on Ni-supported on graphene with the barrier energy of 0.85 eV and a strong exothermicity of 1.45 eV. The key intermediate of O∗C∗CO can be stably chemisorbed on the Ni-supported-graphene surface by riveting two coupled C atoms on the ortho-, meta-, or para-position of graphene six-membered ring, forming four-, five-, and six-membered ring with the carbon atoms of graphene, respectively. Then, the potential energy surfaces of chemisorbed O∗C∗CO hydrogenation indicates that glycol-aldehyde (HOH2C–CHO) would be preferred to form by the kinetically favorable initial C-hydrogenation due to the low rate-limiting barrier of 0.46 eV, while the glyoxal (OHC=CHO) is a considerably competitive product because its rate-limiting barrier is only 0.18 eV higher than that of the glycol-aldehyde. These results suggest that the chainmail catalyst of nickel-supported graphene could be a potential and high-efficient catalyst for synthesis of C2 oxygenates from syngas, which also provides a fundamental insight into the new reaction mechanism of Fischer-Tropsch synthesis.
中文翻译:

机理见解:在Ni负载的石墨烯上通过关键中间体C 2 O 2将合成气直接转化为C 2含氧化合物
合理设计新型催化剂的关键一步,该催化剂可实现从合成气中选择性高效合成C 2+含氧化合物(CO / H 2)是通过费-托合成法(FTS)确定详细的反应机理。在此,报道了两种气态CO单体偶联到化学吸附的乙二酮(O * C * CO)中并随后在镍负载的石墨烯表面的链邮催化剂上将O * C * CO加氢的机理。结果表明,两种气态CO单体可以通过O * CCO单原子化学吸附在负载于石墨烯的镍上的亚稳态O * CCO的中间体,耦合成双原子化学吸附的O * C * CO。 1.45 eV的强烈放热。通过铆接石墨烯六元环的邻位,间位或对位上的两个偶合的C原子,可以稳定地将O ∗ C ∗ CO的关键中间体化学吸附在Ni负载的石墨烯表面上,形成四,分别与具有石墨烯碳原子的五元和六元环。然后,由于0.46 eV的低限速屏障,在动力学上有利的初始C加氢反应会形成2 C–CHO),而乙二醛(OHC = CHO)的竞争性相当强,因为它的限速屏障是仅比乙二醇醛高0.18 eV。这些结果表明,镍负载石墨烯的链邮催化剂可能是一种潜在的高效合成气合成C 2含氧化合物的催化剂,这也为费托合成的新反应机理提供了基础性的见识。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号