Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2021-01-11 , DOI: 10.1016/j.apcatb.2021.119896 Ilaria Lucentini , Germán García Colli , Carlos D. Luzi , Isabel Serrano , Osvaldo M. Martínez , Jordi Llorca
|
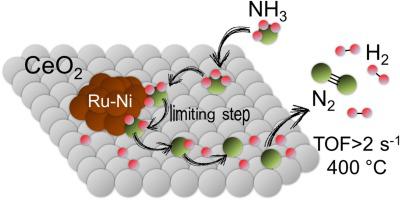
Ceria-supported Ni-Ru bimetallic catalysts with different metal loadings have been prepared by co-impregnation, characterized and tested in the production of hydrogen from the catalytic decomposition of ammonia. The bimetallic catalysts showed an excellent catalytic performance in long-term stability tests with respect to monometallic Ru/CeO2 and Ni/CeO2 and in multicycle tests under pure ammonia. The best catalytic performance has been obtained over catalysts with 2.4−5 wt.% Ni, 0.4−0.6 wt.% Ru, and a Ni/Ru wt.% ratio of ca. 7. TOFH2 values exceeding 2 s−1 have been obtained, which are among the highest reported for ammonia decomposition at 400 °C. Raman spectroscopy, XRD, HRTEM, XPS, TPR and H2 chemisorption have revealed the existence of an intimate contact between Ni and Ru and CeO2, which is considered the reason of the excellent catalytic activity and stability observed. A kinetic model has been developed using the Langmuir-Hinshelwood-Hougen-Watson approach for the decomposition of ammonia in a fixed bed reactor. The reaction rate expression of the ammonia decomposition on Ni-Ru bimetallics supported on ceria suggests that the dehydrogenation of the ammonia adsorbed on the surface of the catalyst is the limiting step of the reaction and that ammonia decomposition is inhibited by the presence of H2.
中文翻译:

CeO 2负载Ni-Ru上催化氨分解制氢:金属负载量和动力学分析的影响
通过共浸渍制备了具有不同金属负载量的二氧化铈负载的Ni-Ru双金属催化剂,对氨催化分解产生的氢气进行了表征和测试。该双金属催化剂在长期稳定性试验中对单金属Ru / CeO 2和Ni / CeO 2以及在纯氨下的多循环试验中均显示出优异的催化性能。相对于具有2.4-5 wt。%的Ni,0.4-0.6 wt。%的Ru和Ni / Ru wt。%的ca的催化剂,获得了最佳的催化性能。7.获得了超过2 s -1的TOF H2值,这是报道的在400°C下氨分解的最高值。拉曼光谱,XRD,HRTEM,XPS,TPR和H 2化学吸附已揭示出Ni与Ru和CeO 2之间存在紧密接触,这被认为是观察到的优异催化活性和稳定性的原因。使用Langmuir-Hinshelwood-Hougen-Watson方法开发了动力学模型,用于在固定床反应器中分解氨。负载在二氧化铈上的Ni-Ru双金属上氨分解的反应速率表示,吸附在催化剂表面的氨的脱氢是反应的限制步骤,并且H 2的存在抑制了氨的分解。































 京公网安备 11010802027423号
京公网安备 11010802027423号