Nano Research ( IF 9.5 ) Pub Date : 2020-11-06 , DOI: 10.1007/s12274-020-3153-6 Kui He , Jiayi Zhu , Lingshan Gong , Yue Tan , Huarui Chen , Huarun Liang , Baihao Huang , Jinbin Liu
|
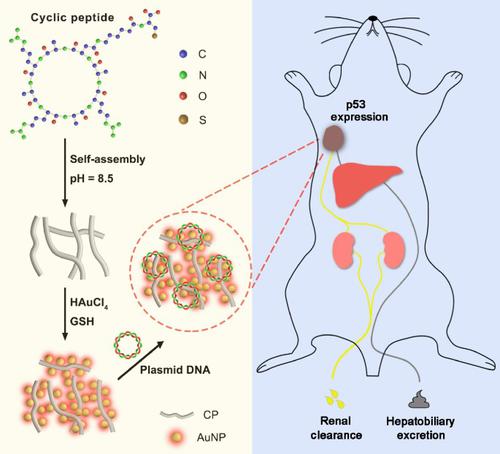
The integration of strong near-infrared (NIR) emission, rapid lysosome escape, fast cellular excretion, and efficient total body clearance is highly desired for nanoparticles (NPs) to achieve synergistic functions in both molecular imaging and delivery. Herein, using a well-designed cyclopeptide (CP) that can spontaneously assemble into controllable nanofibers as template, a facile strategy is reported for in situ self-assembly of NIR-emitting gold NPs (AuNPs) into ordered and well-controlled one-dimensional (1D) nanostructures (AuNPs@CP) with greatly enhanced NIR emission (∼ 6 fold). Comparing with the unassembled AuNPs, the AuNPs@CP are observed to enter living cells through endocytosis, escape from lysosome rapidly, and excrete the cell fast, which shows high gene transfection efficiencies in construction of cell line with ∼ 7.5-fold overexpression of p53 protein. Furthermore, the AuNPs@CP exhibit high in vivo diffusibility and total body clearance efficiency with minimized healthy organ retention, which are also demonstrated to be good nanovectors for plasmid complementary deoxyribonucleic acid 3.1 (pcDNA3.1)(+)-internal ribosome entry site (IRES)-green fluorescent protein (GFP)-p53 plasmid with efficient p53 gene over-expression in tumor site. This facile in situ strategy in fabricating highly luminescent 1D nanostructures provides a promising approach toward future translatable multifunctional nanostructures for delivering, tracking, and therapy.
中文翻译:

将近红外发射金纳米粒子原位自组装为具有快速溶酶体逃逸和快速细胞排泄的身体可清除的一维纳米结构
纳米粒子(NP)强烈希望将强近红外(NIR)发射,快速溶酶体逃逸,快速细胞排泄和有效的全身清除集成在一起,以在分子成像和递送中实现协同功能。在本文中,使用精心设计的可以自发组装成可控纳米纤维的环肽(CP)作为模板,据报导了一种简便的原位策略NIR发射金NP(AuNPs)自组装成有序且受到良好控制的一维(1D)纳米结构(AuNPs @ CP),NIR发射大大增强(约6倍)。与未组装的AuNPs相比,观察到AuNPs @ CP通过内吞作用进入活细胞,迅速从溶酶体逃逸,并快速分泌细胞,这在构建p53蛋白约7.5倍的细胞系中显示出高基因转染效率。 。此外,AuNPs @ CP在体内扩散性和整体清除效率高,健康器官保留量最小,也被证明是质粒互补脱氧核糖核酸3.1(pcDNA3.1)(+)-内部核糖体进入位点的良好纳米载体( IRES)-绿色荧光蛋白(GFP)-p53高效质粒p53基因在肿瘤部位过度表达。这种制造高发光1D纳米结构的简便的原位策略为未来的可翻译多功能纳米结构的传递,跟踪和治疗提供了一种有前途的方法。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号