当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Alkali Metal Carboxylates: Simple and Versatile Initiators for Ring-Opening Alternating Copolymerization of Cyclic Anhydrides/Epoxides
Macromolecules ( IF 5.1 ) Pub Date : 2021-01-07 , DOI: 10.1021/acs.macromol.0c02389 Chong-Min Chen 1 , Xiaowei Xu 2 , He-Yuan Ji 1 , Bin Wang 1 , Li Pan 1 , Yi Luo 2 , Yue-Sheng Li 1
Macromolecules ( IF 5.1 ) Pub Date : 2021-01-07 , DOI: 10.1021/acs.macromol.0c02389 Chong-Min Chen 1 , Xiaowei Xu 2 , He-Yuan Ji 1 , Bin Wang 1 , Li Pan 1 , Yi Luo 2 , Yue-Sheng Li 1
Affiliation
|
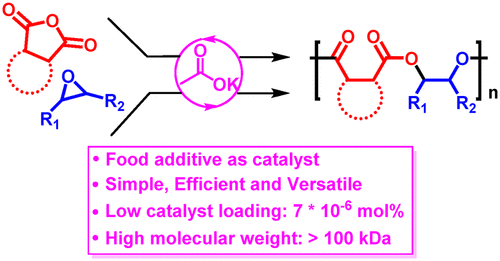
In this contribution, we thoroughly investigated the ring-opening alternating copolymerization (ROAC) of cyclic anhydride and epoxide by using commercially available alkali metal carboxylates (AMCs) as the simple and green initiators. The idea of our work is based on the coordination effects of epoxide on an AMC and the formation of AMC–epoxide adducts, which will weaken the interaction between metal cation and its carboxylate counterion and therefore render the carboxylate to feasibly attack epoxides in a nucleophilic manner at high temperature. The coordination effects of epoxide on the AMC could be proved by Fourier transform infrared (FT-IR) spectroscopy and density functional theory (DFT) calculations. AMCs could effectively catalyze the copolymerization of phthalate anhydride (PA) and cyclohexene oxide (CHO) in bulk at 110 °C, affording polyesters with perfectly alternating structure. Potassium acetate (KOAc) was able to copolymerize some common cyclic anhydrides and epoxide, allowing for the preparation of polyesters with structural diversity. Of note, KOAc could mediate ROAC of PA with propylene oxide (PO) with a high molar feed ratio of [KOAc]/[PA]/[PO] = 1:20 000:150 000, affording poly(PA-alt-PO) with high molecular weight (>100 kDa). Finally, two different polymerization mechanisms, including anionic polymerization and cooperative catalysis, have been proposed according to the interaction strength between metal cation and carboxylate anion. In the “cooperative catalysis” mechanism, the alkali metal cation served as the Lewis acid to activate epoxide and cyclic active species were generated.
中文翻译:

碱金属羧酸盐:简单而通用的引发剂,用于环酐/环氧化合物的开环交替共聚
在这项贡献中,我们通过使用市售的碱金属羧酸盐(AMC)作为简单的绿色引发剂,彻底研究了环状酸酐和环氧化物的开环交替共聚(ROAC)。我们的工作思路是基于环氧化物对AMC的配位作用以及AMC-环氧化物加合物的形成,这将削弱金属阳离子与其羧酸盐抗衡离子之间的相互作用,从而使羧酸盐以亲核方式切实可行地攻击环氧化物在高温下。环氧树脂对AMC的配位作用可以通过傅立叶变换红外光谱(FT-IR)和密度泛函理论(DFT)计算来证明。AMC可在110°C下有效地催化邻苯二甲酸酐(PA)和环己烯氧化物(CHO)的共聚,提供具有完美交替结构的聚酯。乙酸钾(KOAc)能够使一些常见的环状酸酐和环氧化物共聚,从而可以制备结构多样的聚酯。值得注意的是,KOAc可以以高摩尔进料比[KOAc] / [PA] / [PO] = 1:20 000:150 000的环氧丙烷(PO)介导PA的ROAC。高分子量(> 100 kDa)的alt -PO)。最后,根据金属阳离子与羧酸根阴离子之间的相互作用强度,提出了两种不同的聚合机理,包括阴离子聚合和协同催化。在“合作催化”机制中,碱金属阳离子作为路易斯酸来活化环氧化物,并生成了环状活性物质。
更新日期:2021-01-26
中文翻译:

碱金属羧酸盐:简单而通用的引发剂,用于环酐/环氧化合物的开环交替共聚
在这项贡献中,我们通过使用市售的碱金属羧酸盐(AMC)作为简单的绿色引发剂,彻底研究了环状酸酐和环氧化物的开环交替共聚(ROAC)。我们的工作思路是基于环氧化物对AMC的配位作用以及AMC-环氧化物加合物的形成,这将削弱金属阳离子与其羧酸盐抗衡离子之间的相互作用,从而使羧酸盐以亲核方式切实可行地攻击环氧化物在高温下。环氧树脂对AMC的配位作用可以通过傅立叶变换红外光谱(FT-IR)和密度泛函理论(DFT)计算来证明。AMC可在110°C下有效地催化邻苯二甲酸酐(PA)和环己烯氧化物(CHO)的共聚,提供具有完美交替结构的聚酯。乙酸钾(KOAc)能够使一些常见的环状酸酐和环氧化物共聚,从而可以制备结构多样的聚酯。值得注意的是,KOAc可以以高摩尔进料比[KOAc] / [PA] / [PO] = 1:20 000:150 000的环氧丙烷(PO)介导PA的ROAC。高分子量(> 100 kDa)的alt -PO)。最后,根据金属阳离子与羧酸根阴离子之间的相互作用强度,提出了两种不同的聚合机理,包括阴离子聚合和协同催化。在“合作催化”机制中,碱金属阳离子作为路易斯酸来活化环氧化物,并生成了环状活性物质。






























 京公网安备 11010802027423号
京公网安备 11010802027423号