Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2021-01-07 , DOI: 10.1016/j.molstruc.2021.129901 Imane Merimi , Ruby Aslam , Belkheir Hammouti , Tadeusz Szumiata , Hassane Lgaz , Ill-Min Chung
|
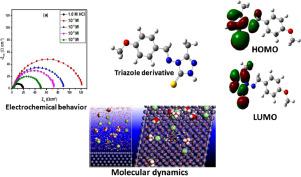
Although the number of reported corrosion inhibitors has increased steeply in recent years, global corrosion damages raised the need for highly effective, new promising compounds for corrosion protection of metals and their alloys. Herein, chemical and electrochemical testing methods like weight loss, electrochemical impedance spectroscopy (EIS) and potentiodynamic polarization (PDP) measurements were applied to evaluate the inhibition performance and adsorption mechanism of (Z)-4-((4-methoxybenzylidene)amino)-5-methyl-2,4-dihydro-3H-1,2,4-triazole-3-thione (2C) against carbon steel (CS) corrosion 1.0 M HCl. Together, electrochemical results and morphological evidence from scanning electron microscope (SEM) demonstrated that, in the presence of 2C, the inhibition efficiency reaches a maximum of 86% at 10−3, forming a compact protective layer on CS surface. On the other hand, corrosion inhibition decreases a little with the rise of the temperature. The investigated compound was classified as a mixed class inhibitor, affecting both anodic and cathodic reactions, while its adsorption followed the Langmuir adsorption isotherm. Molecular dynamics (MD) simulations and quantum chemical calculations were used to give further insights into the inhibiting action of 2C and to explain its mechanism of action. Scanning electron microscopy (SEM) reveals an enhanced surface morphology of carbon steel after the addition of the tested compound to the electrolyte.
中文翻译:

(Z)-4-((4-甲氧基亚苄基)氨基)-5-甲基-2,4-二氢-3H-1,2,4-三唑-3-硫酮对HCl在碳钢上的吸附和抑制机理:实验和理论见解
尽管近年来报告的缓蚀剂的数量急剧增加,但是全球腐蚀破坏引起了对用于金属及其合金防腐蚀的高效,新的有希望的化合物的需求。在这里,化学和电化学测试方法,如重量损失,电化学阻抗谱(EIS)和电位动力学极化(PDP)测量被用来评估对(Z)-4-((4-甲氧基亚苄基)氨基)-的抑制性能和吸附机理。5-甲基-2,4-二氢-3 H -1,2,4-三唑-3-硫酮(2C)对碳钢(CS)腐蚀1.0 M HCl。电化学结果和扫描电镜(SEM)的形态学证据共同表明,在2C存在下,抑制效率在10 -3时最大达到86%,在CS表面形成致密的保护层。另一方面,腐蚀抑制随着温度的升高而略微降低。被研究的化合物被归类为混合类抑制剂,影响阳极和阴极反应,而其吸附遵循Langmuir吸附等温线。分子动力学(MD)模拟和量子化学计算被用来提供2C抑制作用的进一步见解,并解释其作用机理。扫描电子显微镜(SEM)揭示了将测试化合物添加到电解质中后碳钢表面形态的增强。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号